FDA Drug Shortages Frequently Asked Questions
- Why did FDA create a searchable database for drug shortages?
- How often is the information in the database updated?
- Where does the information for the FDA drug shortage list come from?
- Can anyone report a drug shortage?
- What "Shortage Reasons" may appear in the listing for a drug in shortage?
- What does each status mean?
- Can I find all the drugs with a certain status, for example, all the resolved drug shortages?
- What is a "therapeutic category"?
- Does FDA track all drug shortages?
- Why are alternative drugs not provided?
- What do the various dates in the displays mean?
- What do the different values for "Type of Update" mean?
- How long do resolved shortages and discontinuations remain in the list?
- Can I browse the A-Z Index or search by brand names?
- Can I search using a partial name of a drug?
- Can I email or download my search results?
- What is the best way to print a page or a selection from the FDA drug shortages database?
Why did FDA create a searchable database for drug shortages?
FDA created this searchable database to provide stakeholders with easy access to information about drugs in shortage, such as product availability, supply, and estimated duration of shortage. The database includes information about current drugs in shortage, resolved shortages, discontinuations of specific drug products, corresponding therapeutic categories, resource information, and relevant links.- We update the drug shortages searchable database daily.
Where does the information for the FDA drug shortage list come from?
The majority of information is provided to FDA by manufacturers. To ensure the information in this section is current, FDA relies on updates provided by manufacturers on a regular basis. We post updates on drug shortages as soon as we receive them from the manufacturers.Can anyone report a drug shortage?
Yes. Communication between FDA and stakeholders is an essential component of preventing and mitigating drug shortages. While the majority of information in this database comes from the manufacturers, we encourage all stakeholders, including health care professionals, professional groups, and patients, to notify FDA of drug shortage or supply issues.You can send notifications to drug shortage staff via Public Portal Notification
- What "Shortage Reasons" may appear in the listing for a drug in shortage?
According to the Food and Drug Administration Safety and Innovation Act (FDASIA) of 2012 (21 USC 356e.), the drug shortage list should include the following reasons for a shortage:(A) Requirements related to complying with good manufacturing practices.
(B) Regulatory delay.
(C) Shortage of an active ingredient.
(D) Shortage of an inactive ingredient component.
(E) Discontinuation of the manufacture of the drug.
(F) Delay in shipping of the drug.
(G) Demand increase for the drug. - What does each status mean?
Following are definitions for each status:
Currently in Shortage - A situation in which the total supply of all clinically interchangeable versions of an FDA-regulated drug is inadequate to meet the current or projected demand at the user level. In general, the FDA's Drug Shortage Program focuses on shortages of medically necessary products that have a significant effect on public health1.
Resolved - A situation in which the market demand is covered and no supply issues are anticipated by the manufacturers.
Discontinuation - A situation in which a specific drug product is no longer being commercially distributed by an FDA-regulated manufacturer (whether or not a formal withdrawal request is made to the FDA). Note that a discontinuation in the manufacture of a specific drug product does not necessarily create a shortage, because there may be other manufacturers supplying the same or clinically interchangeable products.
Under section 506C of the Federal Food, Drug, and Cosmetic Act, companies are required to notify FDA of a permanent discontinuance of certain drug products, six months in advance, or if that is not possible, as soon as practicable. These drugs include those that are life-supporting, life-sustaining, or for use in the prevention or treatment of a debilitating disease or condition, including any such drug used in emergency medical care or during surgery. The discontinuations listed on FDA's drug shortage database reflect information received from manufacturers and are for informational purposes only.
Can I find all the drugs with a certain status, for example, all the resolved drug shortages?
Yes. In the Current/Resolved Shortages window, use the arrows at the top of the "Status" column to sort and reverse sort the drug names by status. Select the Discontinuations tab to view that status.- Therapeutic category designation is meant to describe labeled indications and populations in which the drug is commonly used, for example Pediatrics. The therapeutic category designation does not indicate FDA approved status. A drug may be designated in more than one therapeutic category. Refer to labeled indications in the package insert for a product's FDA-approved uses.
- FDA's Drug Shortage Program focuses on shortages of medically necessary products since these shortages have the greatest impact on public health. The drug shortage section of FDA's website lists shortages primarily of medically necessary products. Shortages that are expected to be resolved quickly, shortages that involve only a particular strength or package size, and shortages where a substitute strength(s) or package size(s) is available are not usually the focuses of FDA's program.
Why are alternative drugs not provided?
Providing a list of alternative drug products for a drug in shortage is part of the "practice of medicine." FDA does not regulate the practice of medicine. Please refer to The American Society of Health System Pharmacists (ASHP) for lists of drug shortages and suggested alternative therapies.What do the various dates in the displays mean?
- Drugs Currently in Shortage - The date following a company name, for example, "(Reverified 05/19/2014)," is the last time we added, verified or updated the company's information for all the presentations of a particular drug in the drug shortages list.
Example:

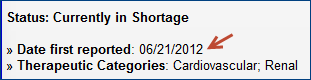
- "Date first reported" is the date FDA received the first notification of a shortage for any presentation of that drug. On the previous website, this date was called "Initial posting date." For some older records, you will see "No date available."
Example:
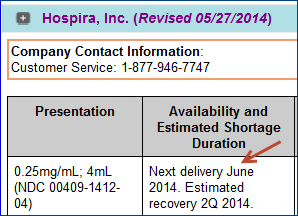
Various dates also appear in the "Availability and Estimated Shortage Duration" column when you open a "Company" panel. For example, "Next delivery" or "Estimated recovery."
Example:
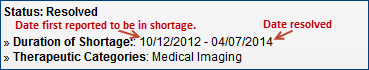
Resolved Drug Shortages - The "Duration of Shortage" date range shows the date the shortage was first reported to FDA and the date the shortage was resolved. If the original reporting date is unavailable, you will see "Prior to 2012" instead.
Example:
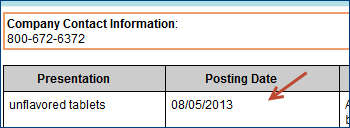
Drugs To Be Discontinued - The "Posting Date" column tells when FDA first listed the company's report of the upcoming discontinuation.
Example:
- Drugs Currently in Shortage - The date following a company name, for example, "(Reverified 05/19/2014)," is the last time we added, verified or updated the company's information for all the presentations of a particular drug in the drug shortages list.
What do the different values for "Type of Update" mean?
A "Type of Update" value applies to all the presentations listed under a certain company.- New: A new drug in shortage
- Revised: Change in information
- Reverified: No change in the information
- Unable to verify: No updates/reverification available from the manufacturer
How long do resolved shortages and discontinuations remain in the list?
Resolved shortages remain on the list for 6 months and discontinued shortages remain on the list for 1 year.Can I browse the A-Z Index or search by brand names?
You can search by some brand names. We use a drug's generic name or active ingredient as the primary listing in the A-Z Index; however, some listings include a brand name in parentheses. An example is "Midazolam Hydrochloride (Versed) Injection," where Versed is a brand name.If you are looking for a brand name, try the search box.
If you don't know the generic name or active ingredient for a brand name drug, you can search the brand name in Drugs@FDA, which will list its active ingredient(s).
Can I search using a partial name of a drug?
Yes, you can search by part of a drug name, as long as you enter at least three characters.For example, to search for "Bismuth Subsalicylate; Metronidazole; Tetracycline Hydrochloride," you could enter any of these and click "Submit":
- bis
- bismuth
- metro
- Tetracycline
- bismuth tetracycline
- The database does not provide either an "email link" feature or the ability to download results into a formatted file for use in a spreadsheet or database application. You can, however, use conventional "copy and paste" functions to grab information to paste into a document or an email message.
What is the best way to print a page or a selection from the FDA drug shortages database?
We recommend using your browser's "Print Preview" function before printing. FDA's printing template creates a footnote for every link on the page. You may be expecting a one-page printout, but end up with several pages.Another way to preview the results before printing is to use the "Print" function to create a PDF document, if that option is available on your computer.
To print all the presentations and company information for a drug, be sure to open all the Company panels before printing.
