|
The following provisions apply to sunscreen products containing aminobenzoic acid, avobenzone, cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, oxybenzone, padimate O, sulisobenzone, titanium dioxide, trolamine salicylate, or zinc oxide, alone or in combination. The provisions do not apply to sunscreen products marketed under approved new drug applications or abbreviated new drug applications.
(a) Principal display panel. In addition to the statement of identity in paragraph (b) of this section, the following labeling shall be prominently placed on the principal display panel:
(1) Effectiveness claim - (i) For products that pass the broad spectrum test in paragraph (j) of this section. (A) The labeling states "Broad Spectrum SPF [insert numerical SPF value resulting from testing under paragraph (i) of this section]".
(B) Prominence. The Broad Spectrum SPF statement shall appear as continuous text with no intervening text or graphic. The entire text shall appear in the same font style, size, and color with the same background color.
(ii) For sunscreen products that do not pass the broad spectrum test in paragraph (j) of this section. The labeling states "SPF [insert numerical SPF value resulting from testing under paragraph (i) of this section]". The entire text shall appear in the same font style, size, and color with the same background color.
(2) Water resistance statements - (i) For products that provide 40 minutes of water resistance according to the test in paragraph (i)(7)(i) of this section. The labeling states "Water Resistant (40 minutes)".
(ii) For products that provide 80 minutes of water resistance according to the test in paragraph (i)(7)(ii) of this section. The labeling states "Water Resistant (80 minutes)".
(b) Statement of identity. The labeling of the product contains the established name of the drug, if any, and identifies the drug as a "sunscreen."
(c) Indications. The labeling of the product states, under the heading "Uses," the phrases listed in this paragraph (c), as appropriate. Other truthful and nonmisleading statements, describing only the uses that have been established and listed in this paragraph (c), may also be used, as provided in § 330.1(c)(2) of this chapter, subject to the provisions of section 502 of the Federal Food, Drug, and Cosmetic Act (the FD&C Act) relating to misbranding and the prohibition in section 301(d) of the FD&C Act against the introduction or delivery for introduction into interstate commerce of unapproved new drugs in violation of section 505(a) of the FD&C Act.
(1) For all sunscreen products, the following indication statement must be included under the heading "Uses": "[Bullet] helps prevent sunburn". See § 201.66(b)(4) of this chapter for definition of bullet.
(2) For sunscreen products with a Broad Spectrum SPF value of 15 or higher according to the tests in paragraphs (i) and (j) of this section, the labeling may include the following statement in addition to the indication in § 201.327(c)(1): "[Bullet] if used as directed with other sun protection measures (see Directions [in bold italic font]), decreases the risk of skin cancer and early skin aging caused by the sun".
(3) Any labeling or promotional materials that suggest or imply that the use, alone, of any sunscreen reduces the risk of or prevents skin cancer or early skin aging will cause the product to be misbranded under section 502 of the FD&C Act (21 U.S.C. 352).
(d) Warnings. The labeling of the product contains the following warnings under the heading "Warnings".
(1) For all sunscreen products. (i) The labeling states "Do not use [bullet] on damaged or broken skin".
(ii) The labeling states "When using this product [bullet] keep out of eyes. Rinse with water to remove."
(iii) The labeling states "Stop use and ask a doctor if [bullet] rash occurs".
(2) For sunscreen products that are broad spectrum with SPF values of at least 2 but less than 15 according to the SPF test in paragraph (i) of this section or that do not pass the broad spectrum test in paragraph (j) of this section. The first statement under the heading "Warnings" states "Skin Cancer/Skin Aging Alert [in bold font]; Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not [in bold font] skin cancer or early skin aging."
(e) Directions. The labeling of the product contains the following statements, as appropriate, under the heading "Directions." More detailed directions applicable to a particular product formulation may also be included.
(1) For all sunscreen products. (i) As an option, the labeling may state "For sunscreen use:".
(ii) The labeling states "[bullet] apply [select one of the following: 'Liberally' or 'generously'] [and, as an option: 'And evenly'] 15 minutes before sun exposure".
(iii) As an option, the labeling may state "[bullet] apply to all skin exposed to the sun".
(iv) The labeling states "[bullet] children under 6 months of age: Ask a doctor".
(2) For sunscreen products with a Broad Spectrum SPF value of 15 or higher according to the tests in paragraphs (i) and (j) of this section. The labeling states "[bullet] Sun Protection Measures. [in bold font] Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: [Bullet] limit time in the sun, especially from 10 a.m.-2 p.m. [bullet] wear long-sleeved shirts, pants, hats, and sunglasses".
(3) For products that satisfy the water resistance test in paragraph (i)(7) of this section. The labeling states "[bullet] reapply: [Bullet] after [select one of the following determined by water resistance test: '40 minutes of' or '80 minutes of'] swimming or sweating [bullet] immediately after towel drying [bullet] at least every 2 hours".
(4) For products that do not satisfy the water resistance test in paragraph (i)(7) of this section. The labeling states "[bullet] reapply at least every 2 hours [bullet] use a water resistant sunscreen if swimming or sweating".
(f) Other information. The labeling of the product contains the following statement under the heading "Other information:" "[bullet] protect the product in this container from excessive heat and direct sun".
(g) False and misleading claims. There are claims that would be false and/or misleading on sunscreen products. These claims include but are not limited to the following: "Sunblock," "sweatproof," and "waterproof." These or similar claims will cause the product to be misbranded under section 502 of the FD&C Act (21 U.S.C. 352).
(h) Labeling of products containing a combination of sunscreen and skin protectant active ingredients. Statements of identity, indications, warnings, and directions for use, respectively, applicable to each ingredient in the product may be combined to eliminate duplicative words or phrases so that the resulting information is clear and understandable. Labeling provisions in § 347.50(e) of this chapter shall not apply to these products.
(i) SPF test procedure - (1) UV source (solar simulator). (i) Emission spectrum. A single port or multiport solar simulator should be filtered so that it provides a continuous emission spectrum from 290 to 400 nanometers (nm) with a limit of 1,500 Watts per square meter (W/m
2) on total irradiance for all wavelengths between 250 and 1,400 nm.
(A) The solar simulator should have the following percentage of erythema-effective radiation in each specified range of wavelengths:
Solar Simulator Emission Spectrum
| Wavelength range (nm)
| Percent erythemal contribution
1
|
|---|
| <290 | <0.1
| | 290-300 | 1.0-8.0
| | 290-310 | 49.0-65.0
| | 290-320 | 85.0-90.0
| | 290-330 | 91.5-95.5
| | 290-340 | 94.0-97.0
| | 290-400 | 99.9-100.0
|
(B) In addition, UVA II (320-340 nm) irradiance should equal or exceed 20 percent of the total UV (290-400 nm) irradiance. UVA I (340-400 nm) irradiance should equal or exceed 60 percent of the total UV irradiance.
(ii) Erythema action spectrum. (A) Calculate the erythema action spectrum weighting factor (Vi) at each wavelength λ:
(1 ) Vi (λ) = 1.0 (250 <λ �298 nm)
(2 ) Vi (λ) = 10
0.094* (
298â??λ) (298 <λ â?¤328 nm)
(3 ) Vi (λ) = 10
0.015* (
140â??λ) (328 <λ â?¤400 nm)
(B) Calculate the erythema-effective UV dose (E) delivered by a solar simulator as follows:
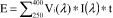
Where Vi(λ) = erythema action spectrum weighting factor at each wavelength λ
I(λ) = irradiance (Watts per square meter) at each wavelength λ
t = exposure time (seconds)
Erythema-effective dose (E) is expressed as effective Joules per square meter (J/m
2-eff).
(C) The emission spectrum must be determined using a handheld radiometer with a response weighted to match the spectrum in ISO 17166 CIE S 007/E entitled "Erythemal reference action spectrum and standard erythema dose," dated 1999 (First edition, 1999-12-15; corrected and reprinted 2000-11-15), which is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. You may obtain a copy from the ISO Copyright Office, Case Postale 56, CH-1211, Geneva 20, Switzerland, telephone +41-22-749-01-11 or fax +41-22-74-09-47. http://www.iso.org. You may inspect a copy at the Center for Drug Evaluation and Research, 10903 New Hampshire Ave., Bldg. 22, Silver Spring, MD 20993, call 301-796-2090, or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html. The solar simulator output should be measured before and after each phototest or, at a minimum, at the beginning and end of each test day. This radiometer should be calibrated using side-by-side comparison with the spectroradiometer (using the weighting factors determined according to paragraph (i)(1)(ii)(A) of this section) at the time of the annual spectroradiometric measurement of the solar simulator as described in paragraph (i)(1)(iv) of this section.
(iii) Operation. A solar simulator should have no significant time-related fluctuations (within 20 percent) in radiation emissions after an appropriate warm-up time and demonstrate good beam uniformity (within 20 percent) in the exposure plane. The delivered dose to the UV exposure site must be within 10 percent of the expected dose.
(iv) Periodic measurement. To ensure that the solar simulator delivers the appropriate spectrum of UV radiation, the emission spectrum of the solar simulator should be measured at least annually with an appropriate and accurately calibrated spectroradiometer system (results should be traceable to the National Institute for Standards and Technology). In addition, the solar simulator must be recalibrated if there is any change in the lamp bulb or the optical filtering components (i.e., filters, mirrors, lenses, collimating devices, or focusing devices). Daily solar simulator radiation intensity should be monitored with a broadband radiometer with a response weighted to match the erythema action spectrum in ISO 17166 CIE S 007/E entitled "Erythemal reference action spectrum and standard erythema dose," which is incorporated by reference in paragraph (i)(1)(ii)(C) of this section. If a lamp must be replaced due to failure or aging during a phototest, broadband device readings consistent with those obtained for the original calibrated lamp will suffice until measurements can be performed with the spectroradiometer at the earliest possible opportunity.
(2) SPF standard - (i) Preparation. The SPF standard should be a formulation containing 7-percent padimate O and 3-percent oxybenzone.
Composition of the Padimate O/oxybenzone SPF Standard
| Ingredients
| Percent by weight
|
|---|
| Part A:
| | | Lanolin | 4.50
| | Cocoa butter | 2.00
| | Glyceryl monostearate | 3.00
| | Stearic acid | 2.00
| | Padimate O | 7.00
| | Oxybenzone | 3.00
| | Part B:
| | | Purified water USP | 71.60
| | Sorbitol solution | 5.00
| | Triethanolamine, 99 percent | 1.00
| | Methylparaben | 0.30
| | Propylparaben | 0.10
| | Part C:
| | | Benzyl alcohol | 0.50
| | Part D:
| | | Purified water USP | QS
1
|
Step 1. Add the ingredients of Part A into a suitable stainless steel kettle equipped with a propeller agitator. Mix at 77 to 82 deg.C until uniform.
Step 2. Add the water of Part B into a suitable stainless steel kettle equipped with a propeller agitator and begin mixing at 77 to 82 deg.C. Add the remaining ingredients of Part B and mix until uniform.
Step 3. Add the batch of Step 1 to the batch of Step 2 and mix at 77 to 82 deg.C until smooth and uniform. Slowly cool the batch to 49 to 54 deg.C.
Step 4. Add the benzyl alcohol of Part C to the batch of Step 3 at 49 to 54 deg.C. Mix until uniform. Continue to cool batch to 35 to 41 deg.C.
Step 5. Add sufficient water of Part D to the batch of Step 4 at 35 to 41 deg.C to obtain 100 grams of SPF standard. Mix until uniform. Cool batch to 27 to 32 deg.C.
(ii) HPLC assay. Use the following high performance liquid chromatography (HPLC) procedure to verify the concentrations of padimate O and oxybenzone in the SPF standard:
(A) Instrumentation. (1 ) Equilibrate a suitable liquid chromatograph to the following or equivalent conditions:
| (i) Column | C-18, 250 millimeters (mm) length, 4.6 mm inner diameter (5 microns)
| | (ii) Mobile Phase | 85:15:0.5 methanol: water: acetic acid
| | (iii) Flow Rate | 1.5 milliliters (mL) per minute
| | (iv) Temperature | Ambient
| | (v) Detector | UV spectrophotometer at 308 nanometers
| | (vi) Attenuation | As needed |
(2 ) Use HPLC grade reagents for mobile phase.
(B) Preparation of the HPLC reference standard. (1 ) Weigh 0.50 gram (g) of oxybenzone USP reference standard into a 250-mL volumetric flask. Dissolve and dilute to volume with isopropanol. Mix well.
(2 ) Weigh 0.50 g of padimate O USP reference standard into a 250-mL volumetric flask. Dissolve and dilute to volume with isopropanol. Mix well.
(3 ) Pipet 3.0 mL of the oxybenzone solution and 7.0 mL of the padimate O solution into a 100-mL volumetric flask. Dilute to volume with isopropanol and mix well.
(C) HPLC system suitability. (1 ) Make three replicate 10-microliter injections of the HPLC reference standard (described in paragraph (i)(2)(ii)(B) of this section). The relative standard deviation in peak areas should not be more than 2.0 percent for either oxybenzone or padimate O.
(2 ) Calculate the resolution (R) between the oxybenzone and padimate O peaks from one chromatogram as follows:
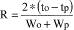
Where to = retention time for oxybenzone
tp = retention time for padimate O
Wo = oxybenzone peak width at baseline
Wp = padimate O peak width at baseline
If the resolution (R) is less than 3.0, adjust the mobile phase or replace the column.
(D) SPF standard assay - (1 ) The SPF standard is diluted to the same concentration as the HPLC reference standard according to the following steps:
(i ) Step 1. Weigh 1.0 g of the SPF standard (described in paragraph (i)(2)(i) of this section) into a 50-mL volumetric flask.
(ii ) Step 2. Add approximately 30 mL of isopropanol and heat with swirling until contents are evenly dispersed.
(iii ) Step 3. Cool to room temperature (15 to 30 deg.C) and dilute to volume with isopropanol. Mix well.
(iv ) Step 4. Pipet 5.0 mL of the preparation into a 50-mL volumetric flask and dilute to volume with isopropanol. Mix well.
(2 )(i ) Inject 10-microliter of diluted SPF standard from paragraph (i)(2)(D)(1 ) of this section and calculate the amount of oxybenzone and padimate O as follows:

(ii ) The percent of oxybenzone and padimate O in the SPF standard should be between 95 and 105.
(3) Test subjects - (i) Number of subjects. A test panel should include enough subjects to produce a minimum of 10 valid test results. A maximum of three subjects may be rejected from this panel based on paragraph (i)(5)(v)) of this section.
(ii) Medical history. (A) Obtain a medical history from each subject with emphasis on the effects of sunlight on the subject's skin. Determine that each subject is in good general health with skin type I, II, or III as follows:
(1 ) Always burns easily; never tans (sensitive).
(2 ) Always burns easily; tans minimally (sensitive).
(3 ) Burns moderately; tans gradually (light brown) (normal).
(4 ) Burns minimally; always tans well (moderate brown) (normal).
(5 ) Rarely burns; tans profusely (dark brown) (insensitive).
(6 ) Never burns; deeply pigmented (insensitive).
(B) Skin type is based on first 30 to 45 minutes of sun exposure after a winter season of no sun exposure. Determine that each subject is not taking topical or systemic medication that is known to alter responses to UV radiation. Determine that each subject has no history of sensitivities to topical products and/or abnormal responses to sunlight, such as a phototoxic or photoallergic response.
(iii) Physical examination. Conduct a physical examination to determine the presence of sunburn, suntan, scars, active dermal lesions, and uneven skin tones on the areas of the back to be tested. A suitable source of low power UVA, such as a Woods lamp, is helpful in this process. If any of these conditions are present, the subject is not qualified to participate in the study. The presence of nevi, blemishes, or moles will be acceptable if, in the physician's judgment, they will neither compromise the study nor jeopardize a subject's safety. Subjects with dysplastic nevi should not be enrolled. Excess hair on the back is acceptable if the hair is clipped. Shaving is unacceptable because it may remove a significant portion of the stratum corneum and temporarily alter the skin's response to UV radiation.
(iv) Informed consent. Obtain legally effective written informed consent from all test subjects.
(4) Sunscreen application. (i) Test site. Test sites are locations on each subject's back, between the beltline and the shoulder blades (scapulae) and lateral to the midline, where skin responses to UV radiation are determined. Responses on unprotected skin (no test material applied) and protected skin (sunscreen test product(s) or SPF standard applied) are determined at separate unprotected and protected test sites, respectively. Test sites should be randomly located in a blinded manner. Each test site should be a minimum of 30 square centimeters and outlined with indelible ink.
(ii) Test subsite. Test subsites are the locations to which UV radiation is administered within a test site. At least five test subsites should receive UV doses within each test site. Test subsites should be at least 0.5 square centimeters (cm
2) in area and should be separated from each other by at least 0.8 cm. Each test subsite should be outlined with indelible ink.
(iii) Applying test materials. Apply the sunscreen test product and the SPF standard at 2 milligrams per square centimeter (mg/cm
2) to their respective test sites. Use a finger cot compatible with the sunscreen to spread the product as evenly as possible.
(iv) Waiting period. Wait at least 15 minutes after applying a sunscreen product before exposing the test sites to UV radiation as described in paragraph (i)(5)) of this section. For water resistant sunscreen products, proceed with the water resistance testing procedure described in paragraph (i)(7) of this section after waiting at least 15 minutes.
(5) UV exposure - (i) Definition of minimal erythema dose (MED). The minimal erythema dose (MED) is the smallest UV dose that produces perceptible redness of the skin (erythema) with clearly defined borders at 16 to 24 hours after UV exposure. The MED for unprotected skin (MEDu) is determined on a test site that does not have sunscreen applied. The MED for protected skin (MEDp) is determined on a test site that has sunscreen applied. An MEDp is determined for the SPF standard (ssMEDp). An MEDp is determined for the sunscreen test product (tpMEDp).
(ii) UV exposure for initial MEDu. For each test subject, administer a series of UV radiation doses expressed as J/m
2-eff (as determined according to paragraph (a)(2) of this section) to the test subsites within an unprotected test site using an accurately calibrated solar simulator. Select doses that are a geometric series represented by 1.25
n (i.e., each dose is 25 percent greater than the previous dose).
(iii) UV exposure for final MEDu, ssMEDp, and tpMEDp. For each subject, determine the final MEDu, ssMEDp, and tpMEDp by administering a series of five UV doses to the appropriate test sites. The middle dose (X) in each of these dose series (i.e., the third dose) should equal the initial MEDu times the expected SPF. Note that the expected SPF equals 1 and 16.3 for the final MEDu and ssMEDp, respectively. The remaining UV doses in the series depend upon the expected SPF value of the sunscreen test product(s).
For products with an expected SPF less than 8, administer UV doses that increase by 25 percent with each successive dose (i.e., 0.64X, 0.80X, 1.00X, 1.25X, and 1.56X). For products with an expected SPF from 8 to 15, administer UV doses that increase by 20 percent with each successive dose (i.e., 0.69X, 0.83X, 1.00X, 1.20X, and 1.44X). For products with an expected SPF higher than 15, administer UV doses that increase by 15 percent with each successive dose (i.e., 0.76X, 0.87X, 1.00X, 1.15X, and 1.32X).
(iv) Evaluation of test subsites. In order that the person who evaluates the test subsites is not biased, he/she should not be the same person who applied the sunscreen drug product to the test site or administered the UV doses. After UV doses are administered, all immediate responses should be recorded. These may include an immediate darkening or tanning, typically grayish or purplish in color, which fades in 30 to 60 minutes; an immediate reddening at the subsite, due to heating of the skin, which fades rapidly; and an immediate generalized heat response, spreading beyond the subsite, which fades in 30 to 60 minutes. After the immediate responses are noted, each subject should shield the exposed area from further UV radiation until the MED is determined. Determine the MED 16 to 24 hours after UV exposure. Because erythema is evaluated 16 to 24 hours after UV exposure, the final MEDu, ssMEDp, and tpMEDp are typically determined the day following determination of the initial MEDu. Evaluate the erythema responses of each test subsite using either tungsten or warm white fluorescent lighting that provides at least 450 lux of illumination at the test site. For the evaluation, the test subject should be in the same position as when the test site was irradiated.
(v) Invalid test data. Reject test data for a test subject if erythema is not present on either the unprotected or protected test sites; or erythema is present at all subsites; or the responses are inconsistent with the series of UV doses administered; or the subject was noncompliant (e.g., the subject withdraws from the test due to illness or work conflicts or does not shield the exposed testing sites from further UV radiation until the MED is determined).
(6) Determination of SPF. (i) Calculate an SPF value for each test subject (SPFi) as follows:
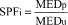
(ii) Calculate the mean
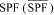
and the standard deviation (s) from the SPFi values. Calculate the standard error (SE), which equals s/â??n (where n equals the number of subjects who provided valid test results). Obtain the t value from Student's t distribution table corresponding to the upper 5-percent point with n - 1 degrees of freedom. Determine the labeled SPF value, which equals the largest whole number less than
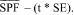
In order for the SPF determination of a test product to be considered valid, the SPF value of the SPF standard should fall within the standard deviation range of the expected SPF (i.e., 16.3 +/-3.43).
(7) Determination of water resistance. The following procedure should be performed in an indoor fresh water pool, whirlpool, and/or hot tub maintained at 23 to 32 deg.C. Fresh water is clean drinking water that meets the standards in 40 CFR part 141. The pool and air temperature and the relative humidity should be recorded.
(i) Water resistance (40 minutes). The labeled SPF should be determined after 40 minutes of water immersion using the following procedure:
(A) Step 1: Apply the sunscreen as described in paragraph (d) of this section.
(B) Step 2: Perform moderate activity in water for 20 minutes.
(C) Step 3: Rest out of water for 15 minutes. Do not towel test site(s).
(D) Step 4: Perform moderate activity in water for 20 minutes.
(E) Step 5: Allow test sites to dry completely without toweling.
(F) Step 6: Apply the SPF standard as described in paragraph (d) of this section.
Step 1. Expose test sites to UV doses as described in paragraph (e) of this section.
(ii) Water resistance (80 minutes). The labeled SPF should be determined after 80 minutes of water immersion using the following procedure:
(A) Step 1: Apply the sunscreen as described in paragraph (d) of this section.
(B) Step 2: Perform moderate activity in water for 20 minutes.
(C) Step 3: Rest out of water for 15 minutes. Do not towel test site(s).
(D) Step 4: Perform moderate activity in water for 20 minutes.
(E) Step 5: Rest out of water for 15 minutes. Do not towel test site(s).
(F) Step 6: Perform moderate activity in water for 20 minutes.
(G) Step 7: Rest out of water for 15 minutes. Do not towel test site(s).
(H) Step 8: Perform moderate activity in water for 20 minutes.
(I) Step 9: Allow test sites to dry completely without toweling.
(J) Step 10: Apply the SPF standard as described in paragraph (d) of this section.
(K) Step 11: Expose test sites to UV doses as described in paragraph (e) of this section.
(j) Broad spectrum test procedure - (1) UV Spectrometry. (i) Plate. Use optical-grade polymethylmethacrylate (PMMA) plates suitable for UV transmittance measurements. The plate should be roughened on one side to a three dimensional surface topography measure (Sa) between 2 and 7 micrometers and must have a rectangular application area of at least 16 square centimeters (with no side shorter than 4 cm).
(ii) Sample holder. The sample holder should hold the PMMA plate in a horizontal position to avoid flowing of the sunscreen drug product from one edge of the PMMA plate to the other. It should be mounted as close as possible to the input optics of the spectrometer to maximize capture of forward scattered radiation. The sample holder should be a thin, flat plate with a suitable aperture through which UV radiation can pass. The PMMA plate should be placed on the upper surface of the sample holder with the roughened side facing up.
(iii) Light source. The light source should produce a continuous spectral distribution of UV radiation from 290 to 400 nanometers.
(iv) Input optics. Unless the spectrometer is equipped with an integrating sphere, an ultraviolet radiation diffuser should be placed between the sample and the input optics of the spectrometer. The diffuser will be constructed from any UV radiation transparent material (e.g., Teflon (R) or quartz). The diffuser ensures that the radiation received by the spectrometer is not collimated. The spectrometer input slits should be set to provide a bandwidth that is less than or equal to 1 nanometer.
(v) Dynamic range of the spectrometer. The dynamic range of the spectrometer should be sufficient to measure transmittance accurately through a highly absorbing sunscreen product at all terrestrial solar UV wavelengths (290 to 400 nm).
(2) Sunscreen product application to PMMA plate. The accuracy of the test depends upon the application of a precisely controlled amount of sunscreen product with a uniform distribution over the PMMA plate. The product is applied at 0.75 mg per square centimeter to the roughened side of the PMMA plate. The sunscreen product should be applied in a series of small dots over the entire PMMA plate and then spread evenly using a gloved finger. Spreading should be done with a very light spreading action for approximately 30 seconds followed by spreading with greater pressure for approximately 30 seconds. The plate should then be allowed to equilibrate for 15 minutes in the dark before the pre-irradiation described in paragraph (c) of this section.
(3) Sunscreen product pre-irradiation. To account for lack of photostability, apply the sunscreen product to the PMMA plate as described in paragraph (b) of this section and then irradiate with a solar simulator described in section 352.70(b) of this chapter. The irradiation dose should be 4 MEDs which is equivalent to an erythemal effective dose of 800 J/m
2 (i.e., 800 J/m
2-eff).
(4) Calculation of mean transmittance values. After pre-irradiation described in paragraph (c) of this section, mean transmittance values should be determined for each wavelength λ over the full UV spectrum (290 to 400 nanometers). The transmittance values should be measured at 1 nanometer intervals. Measurements of spectral irradiance transmitted for each wavelength λ through control PMMA plates coated with 15 microliters of glycerin (no sunscreen product) should be obtained from at least 5 different locations on the PMMA plate [C1(λ), C2(λ), C3(λ), C4(λ), and C5(λ)]. In addition, a minimum of 5 measurements of spectral irradiance transmitted for each wavelength λ through the PMMA plate covered with the sunscreen product will be similarly obtained after pre-irradiation of the sunscreen product [P1(λ), P2(λ), P3(λ), P4(λ), and P5(λ)].
The mean transmittance for each wavelength,
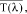
is the ratio of the mean of the C(λ) values to the mean of the P(λ) values, as follows:
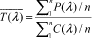
Where n â?¥5
(5) Calculation of mean absorbance values. (i) Mean transmittance values,
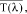
are converted into mean absorbance values,
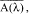
at each wavelength by taking the negative logarithm of the mean transmittance value as follows:
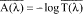
(ii) The calculation yields 111 monochromatic absorbance values in 1 nanometer increments from 290 to 400 nanometers.
(6) Number of plates. For each sunscreen product, mean absorbance values should be determined from at least three individual PMMA plates. Because paragraph (d) of this section requires at least 5 measurements per plate, there should be a total of at least 15 measurements.
(7) Calculation of the critical wavelength. The critical wavelength is identified as the wavelength at which the integral of the spectral absorbance curve reaches 90 percent of the integral over the UV spectrum from 290 to 400 nm. The following equation defines the critical wavelength:
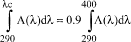
Where λc = critical wavelength
A(λ) = mean absorbance at each wavelength
dλ = wavelength interval between measurements
A mean critical wavelength of 370 nm or greater is classified as broad spectrum protection.
[76 FR 35660, June 17, 2011, as amended at 76 FR 38975, July 5, 2011]
|