Package Insert
Aranespä (Darbepoetin alfa)
Amgen Inc.
Information for Patients and Caregivers
This patient package insert contains information and directions for patients who will be receiving injections of Aranespä and their caregivers. This patient package insert does not include all information about Aranespä. You should discuss any questions about treatment with Aranespä with your doctor.
What is Aranespä?
Aranespä (Air-uh-nesp) is a man made protein that acts like the natural protein human erythropoietin (ee-rith-row-po-eh-tin). Erythropoietin is a hormone,
produced primarily by healthy kidneys, which stimulates the bone marrow to make oxygen-carrying red blood cells.
What is Aranespä used for?
Aranespä is used to treat anemia (a lower than normal number of red blood cells) in patients with kidney disease who may or may not be on dialysis. People with anemia may feel tired or lack energy. Some people may also have shortness of breath, chest pain, and feel cold much of the time.
How does Aranespä work?
When kidneys are not working properly, they cannot produce enough erythropoietin, so the body cannot make enough red blood cells. Aranespä works by stimulating your bone marrow to make more red blood cells. An increase in the number of red blood cells may relieve the symptoms of anemia.
Your doctor will know when Aranespä is working because your blood tests will show an increase in the number of red blood cells. Your doctor may refer to the
results of your blood tests as hemoglobin and/or hematocrit, and your doctor will be checking these tests while you are being treated with Aranespä. The
increase in the number of red blood cells is not immediate; it may take several weeks. The amount of time it takes to reach the red blood cell level that is right for you, and the dose of Aranespä needed to make the red blood cell level rise, is different for each person. You may need several Aranespä dose adjustments
before you reach your correct dose of Aranespä and the correct dose may change over time.
Who should not take Aranespä?
-
People with uncontrolled high blood pressure should not take Aranespä.
-
People who are allergic to other erythropoietins, medicines made using mammalian cells, or any of the ingredients (for example, albumin or polysorbate 80) in Aranespä should not take Aranespä.
Talk to your doctor if you have any questions about this information.
What are the possible or reasonably likely side effects of Aranespä?
Your blood pressure may increase when the number of red blood cells rises, so your doctor or caregiver may monitor your blood pressure more frequently. Some people have also had infections, fevers, headaches, muscle aches or soreness, nausea, and chest pain. If you experience any of these symptoms,
you should call your doctor.
If you are on hemodialysis, there is a risk of blood clots forming at your vascular access. Call your doctor if you think your access is blocked.
Some people experience redness, swelling, or itching at the site of injection. This reaction may be an allergy to the ingredients in Aranespä, or it may be a local irritation. If you notice any signs of redness, swelling, or itching at the site of injection, talk to your doctor.
Serious allergic reactions can also happen. These reactions can cause a rash over the whole body, shortness of breath, wheezing, a drop in blood pressure, swelling around the mouth or eyes, fast pulse, or sweating. If at any time a serious allergic reaction occurs, stop using Aranespä and call your doctor or emergency medical personnel immediately (for example, call 911).
What about pregnancy or breastfeeding?
Aranespä has not been studied in pregnant women and its effects on developing babies are not known. It is also not known if Aranespä can get into human breast milk. If you are pregnant, plan to become pregnant, or think you may be pregnant, or are breastfeeding, you should talk to your doctor before using Aranespä.
What important information do I need to know about taking Aranespä at home?
In some cases, patients may be able to use Aranespä at home. If your doctor has determined that you can safely use Aranespä at home, you and/or your caregiver will receive instructions on how much Aranespä to use, how to inject it, how often it should be injected, and how to dispose of the unused portions of each vial. You should always follow your doctor’s instructions.
Too much Aranespä may cause your body to produce too many red blood cells too fast (lead to a hemoglobin that is too high). Producing too many red blood cells, or producing them too fast may cause serious problems. It is important that your blood pressure be monitored often and to report any changes outside of the guidelines that your doctor has given you, especially if you have heart disease. Certain laboratory tests, such as hemoglobin, hematocrit, or iron level measurements, may also need to be done more often and be reported to your doctor or dialysis center.
Over time, many patients also need to take iron. Your doctor will know when or if you need an iron supplement from your laboratory test results.
Do not change the dose of Aranespä. You should ask your doctor what to do if you miss a dose of Aranespä.
Be sure to change the site for each injection to avoid soreness at any one site. Occasionally a problem may develop at the injection site. If there is a lump, swelling, or bruising at the injection site that does not go away, talk to your doctor.
If you have a hemodialysis vascular access, continue to check the access to make sure it is working. Call your doctor or dialysis center right away if you have any problems or questions.
Always call your doctor if you do not feel well while using Aranespä.
What do you and/or your caregiver need to do to prepare and give an injection of Aranespä?
When you receive your Aranespä, always check to see that:
-
The name Aranespä appears on the package and vial label.
-
The expiration date on the vial label has not passed. You should not use a vial after the date on the label.
-
The strength of the Aranespä vial (number of micrograms [mcg] in the colored dot on the package and on the vial) is the same as the doctor prescribed.
-
The Aranespä liquid in the vial is clear and colorless. Do not use Aranespä
if the liquid in the vial appears discolored or cloudy or if the liquid appears to contain lumps, flakes, or particles.
-
Do not use Aranespä if the yellow cap on the vial has been removed or is missing when the package is opened.
Only use the syringe that your doctor prescribes.
The doctor or nurse will give you instructions on how to measure your dose of Aranespä. This dose will be measured in milliliters. You should only use a syringe that is marked in tenths of milliliters, or mL (for example, 0.2 mL). The doctor or nurse may refer to an “mL” as a “cc” (1 mL = 1 cc). Using an unmarked syringe can lead to a mistake in the dose. If you do not use the correct syringe, you could inject too much or too little Aranespä.
Only use disposable syringes and needles. Use the syringes and needles only once and dispose of them as instructed by your doctor or nurse.
IMPORTANT: TO HELP AVOID POSSIBLE INFECTION, FOLLOW THESE INSTRUCTIONS.
Setting up for an injection
-
Find a clean, flat working surface such as a table.
-
Remove the vial of Aranespä from the refrigerator and place it on your flat working surface; allow it to reach room temperature. This should take about 30 minutes. Do not leave the vial in direct sunlight and do not use a vial that has been frozen.
You should use a vial only once. Do not put the needle through the rubber stopper more than once. DO NOT SHAKE THE VIAL. Shaking may damage the Aranespä. If the Aranespä vial has been shaken vigorously, the solution may appear foamy and it should not be used.
-
Assemble the supplies you will need for an injection:
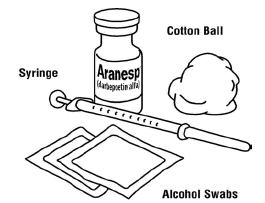
-
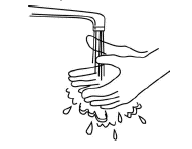
Wash your hands with soap and warm water.
How to prepare the dose of Aranespä
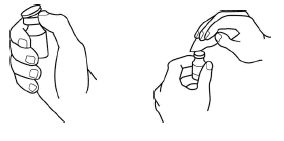
-
Take the yellow color cap off the vial. Clean the rubber stopper with one alcohol swab.
-
Check the package containing the syringe. If the package has been opened or damaged, do not use that syringe. You should dispose of that syringe in the puncture-proof disposal container. If the syringe package is undamaged, open the package and remove the syringe.
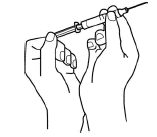
-
Pull the needle cover straight off the syringe. Then, pull back on the plunger to draw air into the syringe. The amount of air drawn into the
syringe should be the same amount (mL or cc) as the dose of Aranespä that your doctor prescribed.
-
Keep the vial on your flat working surface and insert the needle straight down through the rubber stopper.
-
Push the plunger of the syringe down to inject the air from the syringe into the vial of Aranespä.
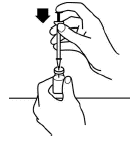
-
Keeping the needle inside the vial, turn the vial upside down. Make sure that the tip of the needle is in the Aranespä liquid.
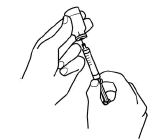
-
Keeping the vial upside down, slowly pull back on the plunger to fill the syringe with Aranespä liquid to the number (mL or cc) that matches the dose your doctor prescribed.
-
Keeping the needle in the vial, check for air bubbles in the syringe. If there are air bubbles, gently tap the syringe with your fingers until the air bubbles rise to the top of the syringe. Then slowly push the plunger up to force the air bubbles out of the syringe. Keep the tip of the needle in the liquid and once again pull the plunger back to the number on the syringe that matches your dose. Check again for air bubbles. The air in the syringe will not hurt you, but too large an air bubble can reduce your dose of Aranespä. If there are still air bubbles, repeat the steps above to remove them.
-
Check again to make sure that you have the correct dose in the syringe. It is important that you use the exact dose prescribed by your doctor.
Selecting and preparing the injection site
-
Choose an injection site. Four recommended injection sites for Aranespä include:
-
The outer area of your upper arms
-
The abdomen (except for the two inch area around your navel)
-
The front of your middle thighs
-
The upper outer areas of your buttocks
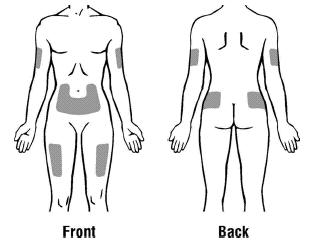
Choose a new site each time you inject Aranespä. Choosing a new site can help avoid soreness at any one site. Do not inject Aranespä into an area that is tender, red, bruised, or hard or that has scars or stretch marks.
-
Clean the injection site with a new alcohol swab.
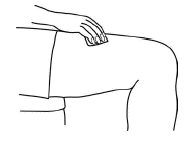
Injecting the dose of Aranespä
For patients not on hemodialysis:
-
Remove the syringe and needle from the vial. Do not lay the syringe down or allow it to touch the surface.
-
Hold the syringe in the hand that you will use to inject Aranespä. Use the other hand to pinch a fold of skin at the cleaned injection site.
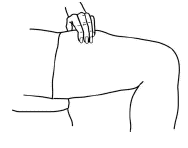
-
Holding the syringe like a pencil, use a quick “dart like” motion to insert the needle either straight up and down (90 degree angle) or at a slight angle (45 degrees) into the skin.
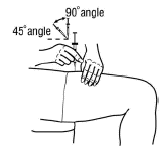
-
After the needle is inserted, let go of the skin. Pull the plunger back slightly. If no blood appears, slowly push the plunger all the way down, until all the Aranespä is injected. If blood comes into the syringe, do not inject Aranespä because the needle has entered a blood vessel. Withdraw the syringe and discard it in the puncture-proof container. Repeat the steps to choose and clean a new injection site and prepare a new syringe.
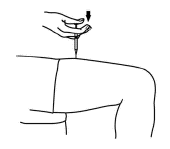
-
When the syringe is empty, pull the needle out of the skin and place a cotton ball or gauze over the injection site and press for several seconds.
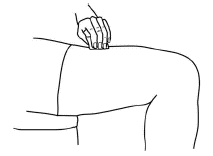
You should ONLY use a syringe and vial once. You should discard the vial with any remaining Aranespä.
For patients on hemodialysis:
-
Clean the venous port of the hemodialysis tubing with a new alcohol swab.
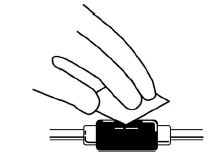
-
Insert the needle of the syringe into the cleaned venous port and push the plunger all the way down to inject all the Aranespä.
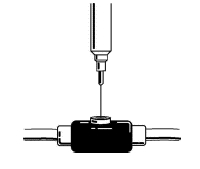
-
Remove the syringe from the venous port and discard the syringe and needle unit in the puncture-proof container (see Disposal of syringes and
needles below).
Disposal of syringes and needles
-
Dispose of the syringe and needle as instructed by your doctor, nurse, or pharmacist, or by following these steps:
-
Do not throw the needle or syringe in the household trash or recycle.
-
DO NOT put the needle cover (the cap) back on the needle. Place the used needle, needle cover, and syringe in a hard plastic container with a screw-on cap or a metal container with a plastic lid, such as a coffee can, labeled “used syringes.” If a metal container is used, cut a small
hole in the plastic lid and tape the lid to the metal container. If a hard plastic container is used, always screw the cap on tightly after each
use.
-
Do not use glass or clear plastic containers.
-
When the container is full, tape around the cap or lid to make sure the cap or lid does not come off.
-
Always keep the container out of the reach of children.
-
You should always first check with your doctor, nurse, or pharmacist for instructions on how to properly dispose of a filled container. There may be special state and local laws for disposal of used needles and syringes. Do not throw the container in household trash. Do not
recycle.
How should Aranespä be stored?
Aranespä should be stored in the refrigerator at 2o to 8oC (36o to 46oF), but not in the freezer. Do not use a vial of Aranespä that has been frozen. Do not leave Aranespä in direct sunlight. Contact your doctor or nurse with any questions about storage. When traveling, transport Aranespä in its dispensing pack in an insulated container with a coolant such as blue ice. To avoid freezing, make sure no Aranespä vial touches the coolant.
[Amgen Logo]
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
Ó2001 Amgen Inc. All rights reserved.
Issue Date: XX/XX/XX
Last Updated: 9/24/2001
Date created: September 26, 2003
 |