|
|
|
|
|||||||
| |||||||||
|
| |||||||||
AVONEX® (Interferon beta-1a)
Table of Contents
Description
DESCRIPTIONAVONEX® (Interferon beta-1a) is a 166 amino acid glycoprotein with a predicted molecular weight of approximately 22,500 daltons. It is produced by recombinant DNA technology using genetically engineered Chinese Hamster Ovary cells into which the human interferon beta gene has been introduced. The amino acid sequence of AVONEX® is identical to that of natural human interferon beta. Using the World Health Organization (WHO) natural interferon beta standard, Second International Standard for Interferon, Human Fibroblast (Gb-23-902-531), AVONEX® has a specific activity of approximately 200 million international units (IU) of antiviral activity per mg of Interferon beta-1a determined specifically by an in vitro cytopathic effect bioassay using lung carcinoma cells (A549) and Encephalomyocarditis virus (ECM). AVONEX® 30 mcg contains approximately 6 million IU of antiviral activity using this method. The activity against other standards is not known. Comparison of the activity of AVONEX® with other Interferon betas is not appropriate, because of differences in the reference standards and assays used to measure activity. AVONEX® is formulated as a sterile, white to off-white lyophilized powder for intramuscular injection after reconstitution with supplied diluent (Sterile Water for Injection, USP). Each 1.0 mL of reconstituted AVONEX® contains 30 mcg of Interferon beta-1a, 15 mg Albumin Human, USP, 5.8 mg Sodium Chloride, USP, 5.7 mg Dibasic Sodium Phosphate, USP, and 1.2 mg Monobasic Sodium Phosphate, USP, at a pH of approximately 7.3.
CLINICAL PHARMACOLOGY
General
Biologic Activities Interferon beta exerts its biological effects by binding to specific receptors on the surface of human cells. This binding initiates a complex cascade of intracellular events that leads to the expression of numerous interferon-induced gene products and markers. These include 2', 5'-oligoadenylate synthetase, b2-microglobulin, and neopterin. These products have been measured in the serum and cellular fractions of blood collected from patients treated with AVONEX®. The specific interferon-induced proteins and mechanisms by which AVONEX® exerts its effects in multiple sclerosis have not been fully defined. Clinical studies conducted in multiple sclerosis patients showed that interleukin 10 (IL-10) levels in cerebrospinal fluid were increased in patients treated with AVONEX® compared to placebo. Serum IL-10 levels were increased 48 hours after intramuscular (IM) injection of AVONEX® and remained elevated for 1 week. However, no relationship has been established between absolute levels of IL-10 and clinical outcome in multiple sclerosis.
Pharmacokinetics After an IM dose, serum levels of AVONEX® typically peak between 3 and 15 hours and then decline at a rate consistent with a 10 hour elimination half-life. Serum levels of AVONEX® may be sustained after IM administration due to prolonged absorption from the IM site. Systemic exposure, as determined by AUC and Cmax values, is greater following IM than subcutaneous (SC) administration. Subcutaneous administration of AVONEX® should not be substituted for intramuscular administration. Subcutaneous and intramuscular administration have been observed to have non-equivalent pharmacokinetic and pharmacodynamic parameters following administration to healthy volunteers. Biological response markers (e.g., neopterin and b2-microglobulin) are induced by AVONEX® following parenteral doses of 15 mcg through 75 mcg in healthy subjects and treated patients. Biological response marker levels increase within 12 hours of dosing and remain elevated for at least 4 days. Peak biological response marker levels are typically observed 48 hours after dosing. The relationship of serum AVONEX® levels or levels of these induced biological response markers to the mechanisms by which AVONEX® exerts its effects in multiple sclerosis is unknown.
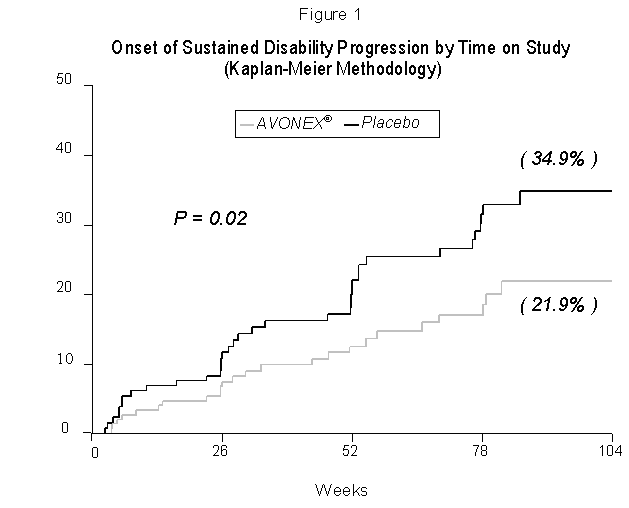
Clinical Studies In Study 1, 301 patients received either 30 mcg of AVONEX® (n=158) or placebo (n=143) by IM injection once weekly. Patients were entered into the trial over a 2˝ year period, received injections for up to 2 years, and continued to be followed until study completion. Two hundred eighty-two patients completed 1 year on study, and 172 patients completed 2 years on study. There were 144 patients treated with AVONEX® for more than 1 year, 115 patients for more than 18 months and 82 patients for 2 years. All patients had a definite diagnosis of multiple sclerosis of at least 1 year duration and had at least 2 exacerbations in the 3 years prior to study entry (or 1 per year if the duration of disease was less than 3 years). At entry, study participants were without exacerbation during the prior 2 months and had Kurtzke Expanded Disability Status Scale (EDSS3) scores ranging from 1.0 to 3.5. Patients with chronic progressive multiple sclerosis were excluded from this study. The primary outcome assessment was time to progression in disability, measured as an increase in the EDSS score of at least 1.0 point that was sustained for at least 6 months. An increase in EDSS score reflects accumulation of disability. This endpoint was used to ensure that progression reflected permanent increase in disability rather than a transient effect due to an exacerbation. Secondary outcomes included exacerbation frequency and results of magnetic resonance imaging (MRI) scans including gadolinium (Gd)-enhanced lesion number and volume and T2-weighted (proton density) lesion volume. Additional secondary endpoints included 2 upper limb (tested in both arms) and 3 lower limb function tests. Twenty-three of the 301 patients (8%) discontinued treatment prematurely. Of these, 1 patient treated with placebo (1%) and 6 patients treated with AVONEX® (4%) discontinued treatment due to adverse events. Thirteen of these 23 patients remained on study and were evaluated for clinical endpoints.
Note: Disability progression represents at least a 1.0 point increase in EDSS score sustained for at least 6 months. Time to onset of sustained progression in disability was significantly longer in patients treated with AVONEX® than in patients receiving placebo (p = 0.02). The Kaplan-Meier plots of these data are presented in Figure 1. The Kaplan-Meier estimate of the percentage of patients progressing by the end of 2 years was 34.9% for placebo-treated patients and 21.9% for AVONEX®-treated patients, indicating a slowing of the disease process. This represents a 37% relative reduction in the risk of accumulating disability in the AVONEX®-treated group compared to the placebo-treated group.
The distribution of confirmed EDSS change from study entry (baseline) to the end of the study is shown in Figure 2. There was a statistically significant difference between treatment groups in confirmed change for patients with at least 2 scheduled visits (136 placebo-treated and 150 AVONEX®-treated patients; p = 0.006; see Table 1). The rate and frequency of exacerbations were determined as secondary outcomes. For all patients included in the study, irrespective of time on study, the annual exacerbation rate was 0.67 per year in the AVONEX®-treated group and 0.82 per year in the placebo-treated group (p = 0.04). AVONEX® treatment significantly decreased the frequency of exacerbations in the subset of patients who were enrolled in the study for at least 2 years (87 placebo-treated patients and 85 AVONEX®-treated patients; p = 0.03; see Table 1). Gd-enhanced and T2-weighted (proton density) MRI scans of the brain were obtained in most patients at baseline and at the end of 1 and 2 years of treatment. Gd-enhancing lesions seen on brain MRI scans represent areas of breakdown of the blood brain barrier thought to be secondary to inflammation. Patients treated with AVONEX® demonstrated significantly lower Gd-enhanced lesion number after 1 and 2 years of treatment (p < 0.05; see Table 1). The volume of Gd-enhanced lesions was also analyzed, and showed similar treatment effects (p < 0.03). Percentage change in T2-weighted lesion volume from study entry to Year 1 was significantly lower in AVONEX®-treated than placebo-treated patients (p = 0.02). A significant difference in T2-weighted lesion volume change was not seen between study entry and Year 2. The exact relationship between MRI findings and the clinical status of patients is unknown. The prognostic significance of MRI findings in these studies have not been evaluated. Of the limb function tests, only 1 demonstrated a statistically significant difference between treatment groups (favoring AVONEX®). A summary of the effects of AVONEX® on the clinical and MRI endpoints of this study is presented in Table 1.
Table 1 Clinical and MRI Endpoints in Study 1
Note: (N: , ) denotes the number of evaluable placebo and AVONEX® patients, respectively.
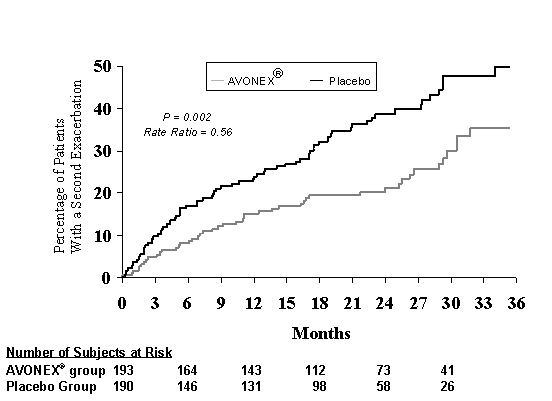
In Study 2, 383 patients who had recently experienced an isolated demyelinating event involving the optic nerve, spinal cord, or brainstem/cerebellum, and who had lesions typical of multiple sclerosis on brain MRI, received either 30 mcg AVONEX® (n = 193) or placebo (n = 190) by IM injection once weekly. All patients received intravenous steroid treatment for the initiating clinical exacerbation. Patients were enrolled into the study over a two-year period and followed for up to three years or until they developed a second clinical exacerbation in an anatomically distinct region of the central nervous system. Sixteen percent of subjects on AVONEX® and 14% of subjects on placebo withdrew from the study for a reason other than the development of a second exacerbation2. Of these early withdrawal patients, 20% treated with placebo and 32% treated with AVONEX® withdrew due to adverse events. The primary outcome measure was time to development of a second exacerbation in an anatomically distinct region of the central nervous system. Secondary outcomes were brain MRI measures, including the cumulative increase in the number of new or enlarging T2 lesions, T2 lesion volume compared to baseline at 18 months, and the number of Gd-enhancing lesions at 6 months. Time to development of a second exacerbation was significantly delayed in patients treated with AVONEX® compared to placebo (p = 0.002). The Kaplan-Meier estimates of the percentage of patients developing an exacerbation within 24 months were 38.6% in the placebo group and 21.1% in the AVONEX® group (Figure 3). The relative rate of developing a second exacerbation in the AVONEX® group was 0.56 of the rate in the placebo group (95% confidence interval 0.38 to 0.81). The brain MRI findings are described in Table 2.
Figure 3
Table 2 Brain MRI Data According to Treatment Group
1 P value <0.001
INDICATIONS AND USAGEAVONEX® (Interferon beta-1a) is indicated for the treatment of patients with relapsing forms of multiple sclerosis to slow the accumulation of physical disability and decrease the frequency of clinical exacerbations. Patients with multiple sclerosis in whom efficacy has been demonstrated include patients who have experienced a first clinical episode and have MRI features consistent with multiple sclerosis. Safety and efficacy in patients with chronic progressive multiple sclerosis have not been established.
CONTRAINDICATIONSAVONEX® is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta, human albumin, or any other component of the formulation.
WARNINGSAVONEX® should be used with caution in patients with depression or other mood disorders, conditions that are common with multiple sclerosis. Depression and suicide have been reported to occur with increased frequency in patients receiving interferon compounds, including AVONEX® . Patients treated with AVONEX® should be advised to report immediately any symptoms of depression and/or suicidal ideation to their prescribing physicians. If a patient develops depression or other severe psychiatric symptoms, cessation of AVONEX® therapy should be considered. In Study 2, AVONEX®-treated patients were more likely to experience depression than placebo-treated patients. An equal incidence of depression was seen in the placebo-treated and AVONEX® -treated patients in Study 1. Additionally, there have been post-marketing reports of depression, suicidal ideation and/or development of new or worsening of pre-existing other psychiatric disorders, including psychosis. Some of these patients improved upon cessation of AVONEX® dosing. Anaphylaxis has been reported as a rare complication of AVONEX® use. Other allergic reactions have included dyspnea, orolingual edema, skin rash and urticaria (see ADVERSE REACTIONS). Decreased Peripheral Blood Counts Decreased peripheral blood counts in all cell lines, including rare pancytopenia and thrombocytopenia, have been reported from post-marketing experience (see ADVERSE REACTIONS). Some cases of thrombocytopenia have had nadirs below 10,000/µl. Some cases reoccur with rechallenge (see ADVERSE REACTIONS). Patients should be monitored for signs of these disorders (see Precautions: Laboratory Tests). Albumin (Human) This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have been identified for albumin.
PRECAUTIONS
Seizures
Cardiomyopathy and Congestive Heart Failure
Autoimmune Disorders
Hepatic Injury
Information to Patients Patients should be informed of the most serious ( see WARNINGS) and the most common adverse events associated with AVONEX® administration, including symptoms associated with flu syndrome (see ADVERSE REACTIONS). Symptoms of flu syndrome are most prominent at the initiation of therapy and decrease in frequency with continued treatment. Concurrent use of analgesics and/or antipyretics may help ameliorate flu-like symptoms on treatment days. Patients should be cautioned to report depression or suicidal ideation (see WARNINGS). Patients should be advised about the abortifacient potential of AVONEX® (see Precautions: Pregnancy - Teratogenic Effects). When a physician determines that AVONEX® can be used outside of the physician's office, persons who will be administering AVONEX® should receive instruction in reconstitution and injection, including the review of the injection procedures. If a patient is to self-administer, the physical ability of that patient to self-inject intramuscularly should be assessed. The first injection should be performed under the supervision of a qualified health care professional. A puncture-resistant container for disposal of needles and syringes should be used. Patients should be instructed in the technique and importance of proper syringe and needle disposal and be cautioned against reuse of these items.
Laboratory Tests
Drug Interactions
Carcinogenesis, Mutagenesis, and Impairment of Fertility Mutagenesis: AVONEX® was not mutagenic when tested in the Ames bacterial test and in an in vitro cytogenetic assay in human lymphocytes in the presence and absence of metabolic activation. These assays are designed to detect agents that interact directly with and cause damage to cellular DNA. AVONEX® is a glycosylated protein that does not directly bind to DNA. Impairment of Fertility: No studies were conducted to evaluate the effects of AVONEX® on fertility in normal women or women with multiple sclerosis. It is not known whether AVONEX® can affect human reproductive capacity. Menstrual irregularities were observed in monkeys administered AVONEX® at a dose 100 times the recommended weekly human dose (based upon a body surface area comparison). Anovulation and decreased serum progesterone levels were also noted transiently in some animals. These effects were reversible after discontinuation of drug. Treatment of monkeys with AVONEX® at 2 times the recommended weekly human dose (based upon a body surface area comparison) had no effects on cycle duration or ovulation. The accuracy of extrapolating animal doses to human doses is not known. In the placebo-controlled studies in multiple sclerosis, 5% of patients receiving placebo and 6% of patients receiving AVONEX® experienced menstrual disorder. If menstrual irregularities occur in humans, it is not known how long they will persist following treatment.
Pregnancy - Teratogenic Effects
Nursing Mothers
Pediatric Use
Geriatric Use
ADVERSE REACTIONSDepression, suicidal ideation, and new or worsening other psychiatric disorders have been observed to be increased in patients using interferon compounds including AVONEX® (see WARNINGS: Depression and Suicide). Anaphylaxis and other allergic reactions have been reported in patients using AVONEX® ( see WARNINGS: Anaphylaxis). Decreased peripheral blood counts have been reported in patients using AVONEX® (see WARNINGS: Decreased Peripheral Blood Counts). Seizures, cardiovascular adverse events, and autoimmune disorders also have been reported in association with the use of AVONEX® (see Precautions). The adverse reactions most commonly reported in patients associated with the use of AVONEX® were flu-like and other symptoms occurring within hours to days following an injection. Symptoms can include myalgia, fever, fatigue, headaches, chills, nausea, and vomiting. Some patients have experienced paresthesias, hypertonia and myasthenia. The most frequently reported adverse reaction resulting in clinical intervention (e.g., discontinuation of AVONEX® , or the need for concomitant medication to treat an adverse reaction symptom) were flu-like symptoms and depression. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of AVONEX® cannot be directly compared to rates in clinical trials of other drugs and may not reflect the rates observed in practice. The data described below reflect exposure to AVONEX® in 351 patients, including 319 patients exposed for 6 months, and 288 patients exposed for greater than one year in placebo-controlled trials. The mean age of patients receiving AVONEX® was 35 years, 74% were women and 89% were Caucasian. Patients received either 30 mcg AVONEX® or placebo. Table 3 enumerates adverse events and selected laboratory abnormalities that occurred at an incidence of at least 2% higher frequency in AVONEX®-treated subjects than was observed in the placebo group. Reported adverse events have been classified using standard COSTART terms.
Table 3 Adverse Events and Selected Laboratory Abnormalities in the Placebo-Controlled Studies
No AVONEX®-treated patients attempted suicide in the two placebo-controlled studies. In Study 2, AVONEX®-treated patients were more likely to experience depression than placebo-treated patients (20% in AVONEX® group vs. 13% in placebo group). The incidences of depression in the placebo-treated and AVONEX®-treated patients in Study 1 were similar. In Study 1, suicidal tendency was seen more frequently in AVONEX®-treated patients (4% in AVONEX® group vs. 1% in placebo group) ( see WARNINGS).
Seizures
Post Marketing Experience Infrequent reports of congestive heart failure, cardiomyopathy, and cardiomyopathy with congestive heart failure with rare cases being temporally related to the administration of AVONEX® (see Precautions: Cardiomyopathy and Congestive Heart Failure). Decreased peripheral blood counts in all cell lines, including rare pancytopenia and thrombocytopenia (see WARNINGS: Decreased Peripheral Blood Counts). Some cases of thrombocytopenia have had nadirs below 10,000/µl. Some of these cases reoccur upon rechallenge. Hepatic injury including elevated serum hepatic enzyme levels and hepatitis, some of which have been severe, has been reported post-marketing (see Precautions: Hepatic Injury). Meno- and metrorrhagia have also been reported in post-marketing experience. Because reports of these reactions are voluntary and the population is of an uncertain size, it is not always possible to reliably estimate the frequency of the event or establish a causal relationship to drug exposure.
Adverse Reactions Associated with Subcutaneous Use
Immunogenicity These data reflect the percentage of patients whose test results were considered positive for antibodies to AVONEX® using a two-tiered assay (ELISA binding assay followed by an antiviral cytopathic effect assay), and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of neutralizing activity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to AVONEX® with the incidence of antibodies to other products may be misleading. Anaphylaxis has been reported as a rare complication of AVONEX® use. Other allergic reactions have included dyspnea, orolingual edema, skin rash and urticaria see WARNINGS: Anaphylaxis).
DRUG ABUSE AND DEPENDENCEThere is no evidence that abuse or dependence occurs with AVONEX® therapy. However, the risk of dependence has not been systematically evaluated.
OVERDOSAGESafety of doses higher than 60 mcg once a week have not been adequately evaluated. The maximum amount of AVONEX® that can be safely administered has not been determined.
DOSAGE AND ADMINISTRATIONThe recommended dosage of AVONEX® (Interferon beta-1a) is 30 mcg injected intramuscularly once a week. AVONEX® is intended for use under the guidance and supervision of a physician. Patients may self-inject only if their physician determines that it is appropriate and with medical follow-up, as necessary, after proper training in intramuscular injection technique. Use appropriate aseptic technique during the preparation of AVONEX® . To reconstitute lyophilized AVONEX® , use a sterile syringe and MICRO PIN® to inject 1.1 mL of the supplied diluent , Sterile Water for Injection, USP, into the AVONEX® vial. Gently swirl the vial of AVONEX® to dissolve the drug completely. DO NOT SHAKE. The reconstituted solution should be clear to slightly yellow and without particles. Inspect the reconstituted product visually prior to use. Discard the product if it contains particulate matter or is discolored. Each vial of reconstituted solution contains 30 mcg Interferon beta -1a. Withdraw 1.0 mL of reconstituted solution from the vial into a sterile syringe fitted with a 23-Ľ gauge needle and inject the solution intramuscularly. Sites for injection include the thigh or upper arm. The AVONEX® and diluent vials are for single-use only; unused portions should be discarded. (See Medication Guide for self-injection procedure.)
HOW SUPPLIEDAVONEX® is supplied as a lyophilized powder in a single-use vial containing 33 mcg (6.6 million IU) of Interferon beta-1a, 16.5 mg Albumin Human, USP, 6.4 mg Sodium Chloride, USP, 6.3 mg Dibasic Sodium Phosphate, USP, and 1.3 mg Monobasic Sodium Phosphate, USP, and is preservative-free. Diluent is supplied in a single-use vial (Sterile Water for Injection, USP). AVONEX® is available in the following package configuration (NDC 59627-001-03): Package (Administration Pack) containing four Administration Dose Packs (each containing one vial of AVONEX®, one 10 mL diluent vial, two alcohol wipes, one gauze pad, one 3 mL syringe, one Micro Pin®* vial access pin, one 23-Ľ gauge needle, and one adhesive bandage).
Stability and Storage
REFERENCES
1 Jacobs LD, et al, Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis.
2 Jacobs LD, et al, Intramuscular interferon beta-1a initiated during a first demyelinating event in multiple sclerosis.
3 Kurtzke JF, Rating neurolgic impairment in multiple sclerosis: an expanded disability status scale (EDSS).
AVONEX® (Interferon beta-1a)
U.S. Patent Pending Rx only *Micro Pin® is the trademark of B. Braun Medical Inc.
Last Updated: 2/14/2003
Date created: September 26, 2003
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||