Drugs@FDA Frequently Asked Questions
- What are the main uses of Drugs@FDA?
- What products are in Drugs@FDA?
- What products are not in Drugs@FDA?
- Why doesn't Drugs@FDA include dietary supplements?
- What information is typically available for a product in Drugs@FDA?
- How can I search Drugs@FDA?
- How do searches work in Drugs@FDA?
- How can I find out if a generic drug is available for a brand-name drug that is approved under a New Drug Application (NDA)?
- How often do you update Drugs@FDA?
- Where does the information in Drugs@FDA come from?
- How does Drugs@FDA compare with the Orange Book?
- How does Drugs@FDA compare with the Purple Book?
- How are BLAs that were formerly approved under an NDA and subsequently deemed a BLA on March 23, 2020, displayed on drugs@FDA?
- What do the submission classification codes for NDAs and review designation codes stand for?
- Can I get a copy of the Drugs@FDA database?
- How can I get further assistance?
1. What are the main uses of Drugs@FDA?
You can use Drugs@FDA to find:
- FDA-approved labeling for products for human use*
- Therapeutically equivalent drug products, including generic drug products
- Product information for patients
- FDA-approved products* with a specific active ingredient
- A product’s approval history, including approval letters and FDA reviews about the safety and effectiveness of the product
* For details about the scope of FDA-approved products for human use in Drugs@FDA, see What products are in Drugs@FDA? and What products are not in Drugs@FDA?.
2. What products are in Drugs@FDA?
Drugs@FDA contains information about products approved by the FDA for human use in the United States since 1938, including:
- Prescription products:
- Brand-name drug products (for example, CRESTOR, LESCOL XL, LIPITOR, LIVALO, PRAVACHOL, ZOCOR), including discontinued drugs and drug products approved under "Submission Classification ‘Type 6’"
- Generic drug products (for example, atorvastatin*), including discontinued drugs
- Many therapeutic biological products (for example, CIMZIA, ENBREL, HUMIRA, REMICADE, and SIMPONI), including biosimilar products (for example, AMJEVITA, CYLTEZO, HADLIMA, and HYRIMOZ). (See biological products that are not included in Drugs@FDA under the heading “What products are not in Drugs@FDA?”)
- Medical gases
- Over-the-counter (OTC) drugs:
- Brand-name drugs (for example, ADVIL, MOTRIN)
- Generic drugs (for example, ibuprofen*)
* Use the term "atorvastatin" when searching for "atorvastatin calcium tablets" and use the term "ibuprofen" when searching for "ibuprofen tablets."
3. What products are not in Drugs@FDA?
Drugs@FDA does not include information about the following FDA-approved products for human use, which are regulated by the Center for Biologics Evaluation and Research:
- Vaccines
- Allergenic products (for example, allergen extracts for diagnosis and treatment)
- Blood and blood products intended for infusion
- Plasma derivatives [for example, Albumin, Immune Globulin Intravenous (IGIV)]
- Cellular and gene therapy products (for example, KYMRIAH, MACI, YESCARTA, ZOLGENSMA)
- Products regulated by CBER under an NDA or an ANDA (for example, Plasma Volume Expanders, Platelet Additive Solutions)
Drugs@FDA does not include information about the following FDA-regulated products that do not require FDA approval to be sold in the United States:
- OTC drug products marketed under the OTC drug monograph system. These products do not have applications (for example, bacitracin zinc ointment, diphenhydramine hydrochloride tablets.)
- Dietary supplements (for example, St. John's wort, vitamin E)
Drugs@FDA also does not include information about the following products:
- Animal prescription and OTC drugs. See Animal Drugs@FDA.
- Drug and biological products sold outside the United States that are not approved for marketing in the United States
- Drug and biological products under review at FDA for approval (investigational products)
4. Why doesn't Drugs@FDA include dietary supplements?
Dietary supplements do not require FDA approval to be sold in the United States. (Drugs@FDA only includes information about FDA-approved drugs.) FDA's Center for Food Safety and Applied Nutrition is responsible for FDA's oversight of dietary supplements.
5. What information is typically available for a product in Drugs@FDA?
The following information is typically available for a product in Drugs@FDA:
- Drug name(s), active ingredient(s), dosage form(s), route(s) of administration, and strength(s)
- The latest FDA-approved labeling and previously approved labeling:
- For prescription products, Drugs@FDA typically includes the Prescribing Information and FDA-approved patient labeling when available (for example, Medication Guides, Patient Information, and/or Instructions for Use), and Drugs@FDA may include FDA-approved carton/container labeling
- For OTC drugs, Drugs@FDA typically includes the carton/container labeling (for example, Drug Facts)
- Regulatory information including:
- Application type: New Drug Application (NDA), Biologics License Application (BLA), Abbreviated New Drug Application (ANDA)
- Application number
- Company that sponsored the application
- Approval letters and approval dates
- Marketing status (for example, prescription, OTC, discontinued)
- Submission classification
- FDA and sponsor meeting minutes regarding the development of the product
- Reference Listed Drug (RLD) and Reference Standard (RS) information
- Therapeutic Equivalence Codes (TE Codes)
- Therapeutically equivalent drug products
- Reviews by FDA staff that evaluate the safety and effectiveness of the product and a list of FDA staff involved in the review of the application
The majority of labeling, approval letters, FDA staff reviews, and other information listed above are available for FDA-approved prescription drug and biological products for human use since 1998 (see the exceptions under “What products are not in Drugs@FDA"?).
6. How can I search Drugs@FDA?
You can search Drugs@FDA in the following ways:
- Use the search box on the home page to search by:
- Drug name(s)
- Active ingredient(s)
- Application number (NDA, ANDA, or BLA number)
- Browse by drug name (in alphabetical order) using the A-Z Index.
Unlike the search box results, the A-Z "Drug Name" search results for an active ingredient will not include brand name drugs for this active ingredient or drugs that contain this active ingredient and other active ingredient(s). For example, the search results for "LISINOPRIL" (using the A-Z "Drug Name" search) will not include PRINIVIL, ZESTRIL, or QBRELIS and will not include ZESTORETIC (lisinopril and hydrochlorothiazide tablets). - Use the "Drug Approval Reports by Month" menus on the Drugs@FDA home page to find the following information by month:*
- All approvals and tentative approvals
- Original NDA and original BLA approvals
- Original ANDA approvals
- Supplemental approvals to NDAs and BLAs
- Tentative ANDA approvals
* For details about the scope of FDA-approved products for human use in Drugs@FDA, see "What products are in Drugs@FDA?" and "What products are not in Drugs@FDA?."
7. How do searches work in Drugs@FDA?
For a drug name or active ingredient search:
- You can type in all or part of the drug or active ingredient name.
- You must enter at least three characters.
- When you enter a partial-word search, Drugs@FDA searches for that string of characters (in the exact order you typed them) anywhere in a drug or active ingredient name. For example, if you enter "proz" you will retrieve a list of drugs that have that four-letter string somewhere in the brand name or somewhere in the active ingredient name:
- CEFPROZIL (an active ingredient)
- OXAPROZIN (an active ingredient)
- PROZAC (a brand-name drug)
- PROZAC WEEKLY (a brand-name drug)
- CEFZIL (CEFPROZIL is the active ingredient)
- DAYPRO (OXAPROZIN is the active ingredient)
- DAYPRO ALTA (OXAPROZIN is the active ingredient)
If you enter two words separated by a space, Drugs@FDA will look for records containing both words, whether they occur next to or apart from each other, within the drug name or active ingredient name (shown inside parentheses). For example, if you enter "chlorpheniramine pseudoephedrine" you will retrieve:
- ADVIL ALLERGY SINUS (CHLORPHENIRAMINE MALEATE; IBUPROFEN; PSEUDOEPHEDRINE HYDROCHLORIDE)
- CHILDREN'S ADVIL ALLERGY SINUS (CHLORPHENIRAMINE MALEATE; IBUPROFEN; PSEUDOEPHEDRINE HYDROCHLORIDE)
- CHLOR-TRIMETON (CHLORPHENIRAMINE MALEATE; PSEUDOEPHEDRINE SULFATE)
- CODIMAL-L.A. 12 (CHLORPHENIRAMINE MALEATE; PSEUDOEPHEDRINE HYDROCHLORIDE)
- HYDROCODONE BITARTRATE, CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE (CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE)
- ISOCLOR (CHLORPHENIRAMINE MALEATE; PSEUDOEPHEDRINE HYDROCHLORIDE)
- PSEUDOEPHEDRINE HYDROCHLORIDE AND CHLORPHENIRAMINE MALEATE (CHLORPHENIRAMINE MALEATE; PSEUDOEPHEDRINE HYDROCHLORIDE)
- ZUTRIPRO (CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE)
For application number searches:
- You must enter all the digits for the NDA, ANDA, or BLA number.
- You must enter three to six digits.
For example:
- NDA:
- 211349
- 20702 or 020702
- 7513, 07513, or 007513
- 004, 0004, 00004, or 000004
- ANDA:
- 211058
- 71617 or 071617
- BLA:
- 761112
You cannot combine an application number with a drug or active ingredient name in a search (for example, searching for "fluoxetine 18936" will not return any results).
Note:The drugs that are listed on the "Search Results" page are not always related in terms of their chemical makeup or the conditions they treat and are not likely substitutable. They appear together because their brand names or active ingredient names contain the words you entered in the search box. Even if drugs have the same active ingredient, dosage form, and strength, it might not be safe to use one drug in place of the other. You should always consult with a healthcare provider to determine if one drug can be safely substituted for another.
8. How can I find out if a generic drug is available for a brand-name drug that is approved under a New Drug Application (NDA)?
Drugs@FDA can tell you if there is a therapeutic equivalent for a prescription innovator drug or prescription generic drug.
- On the home page, search by the name of the brand-name or generic drug.
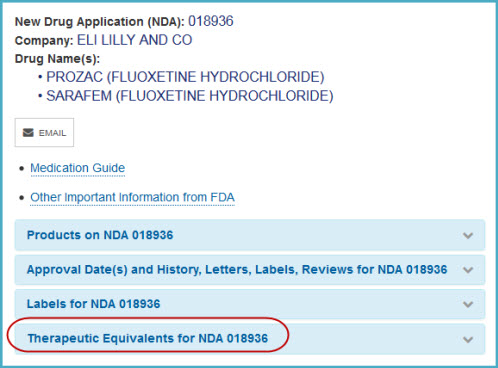
- On the Search Results page, select the drug name. A panel will open and list one or more applications with the same drug name.
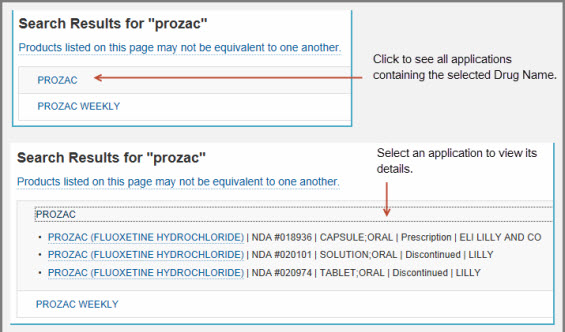
- If one or more generic drugs are available, you will see a panel titled "Therapeutic Equivalents for NDA xxxxxx.” Click on the panel to see the therapeutically equivalent drug products, including generic drugs.
9. How often do you update Drugs@FDA?
We update the Drugs@FDA database every day (sometimes several times throughout the day) with new information about approved drug and biological products. We typically add information to Drugs@FDA about approved drug and biological products within two business days of approval. This may include information from original NDAs/BLAs, efficacy supplements, or labeling supplements.
10. Where does the information in Drugs@FDA come from?
The information in Drugs@FDA comes from:
- Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book)
- The Document Archiving, Reporting, and Regulatory Tracking System (DARRTS) and other internal systems used by FDA staff to track information about approved applications, approved supplements, and tentative approvals.
11. How does Drugs@FDA compare with the Orange Book?
Drugs@FDA and the Orange Book share some content and retrieval capabilities, but Drugs@FDA does not replace the Orange Book. You can read about the origin and purpose of the Orange Book in the Orange Book Preface.
Drugs@FDA includes information and features that are not in the Orange Book:
- Tentative Approvals and "Type 6" approvals
- Labeling, approval letters, FDA and sponsor meeting minutes regarding the development of the product, safety information, and reviews from FDA staff about the safety and effectiveness of the product
- Therapeutic biological products (regulated under BLAs) approved by CDER
- Tables of therapeutically equivalent products
- Tables of OTC drugs containing the same active ingredient, strength, dosage form, and route of administration
- Drug approval reports by month
The Orange Book includes information and features that are not in Drugs@FDA:
- Patent and exclusivity information
- In the Orange Book, you can search by:
- applicant (company)
- dosage form (for example, tablet, capsule)
- route of administration (for example, oral, subcutaneous, intravenous)
- patent number
- In the Orange Book, you can filter search results by prescription (Rx), OTC, and discontinued drugs.
12. How does Drugs@FDA compare with the Purple Book?
Drugs@FDA and the Purple Book share some content and retrieval capabilities for products regulated by the Center for Drug Evaluation and Research (CDER), but Drugs@FDA does not replace the Purple Book. You can learn more about the Purple Book and its use on the About Purple Book webpage.
Drugs@FDA includes information and features that are not in the Purple Book:
- Labeling, approval letters, and information and reviews from FDA staff about the safety, purity and potency (safety and effectiveness) of the product
- "Type 6," "Type 9," or "Type 10" approvals
- Drugs approved under NDAs
The Purple Book includes information and features for biological products regulated by CDER that are not in Drugs@FDA:
- Exclusivity information
- The Purple Book has simple and advanced search capabilities and you can search by:
- proprietary (brand) name
- nonproprietary (proper) name
- applicant (company)
- dosage form (for example, injection)
- product presentation (for example auto injector, pre-filled syringe, vial)
- route of administration (for example, subcutaneous, intravenous)
- strength
- In the Purple Book, you can sort search results using more than 20 different properties including, licensed, revoked, name, applicant, product presentation, BLA number, etc.
- The Purple Book also includes FDA-licensed biological products regulated by the Center for Biologics Evaluation and Research
13. How are BLAs that were formerly approved under an NDA and subsequently deemed a BLA on March 23, 2020, displayed on drugs@FDA?
On March 23, 2020, an approved application for a biological product under section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) was deemed to be a license for the biological product under section 351 of the Public Health Service Act (PHS Act), as required by section 7002(e)(4)(A) of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). If an application for a biological product was submitted under section 505 of the FD&C Act, filed not later than March 23, 2019, and not approved as of March 23, 2020, section 7002(e)(4)(B) of the BPCI Act (added by section 607 of the Further Consolidated Appropriations Act, 2020) provides that if such an application is approved under section 505 of the FD&C Act before October 1, 2022, it will be deemed to be a license for the biological product under section 351 of the PHS Act upon approval.
Information about a product(s) approved in an NDA that was deemed to be a BLA continues to be included in Drugs@FDA. FDA assigned the same application number used for the approved NDA to the deemed BLA, so a search by "application number" will continue to lead to information on the application; however, the corresponding application type has changed from "NDA" to "BLA." The Drugs@FDA entry for an NDA that was deemed to be a BLA includes information related to the initial approval of the application under section 505 of the FD&C Act prior to the approved application being deemed to be a BLA.
Drugs@FDA no longer includes information on an application for a biological product that was approved under section 505 of the FD&C Act in circumstances in which the application holder notified FDA in writing that the product(s) was no longer marketed and requested that FDA withdraw approval of the application, and such withdrawal of approval was announced in a Federal Register notice with an effective date on or before March 23, 2020. For a list of withdrawn applications for biological products, see https://www.fda.gov/drugs/guidance-compliance-regulatory-information/deemed-be-license-provision-bpci-act.
For additional information, see FDA's guidance on The "Deemed to Be a License" Provision of the BPCI Act: Questions and Answers
14. What do the submission classification codes for NDAs and Review Designation codes stand for?
NDA classification codes, shown below, provide a way of categorizing NDAs to promote consistency across review divisions, enable analysis of trends, and facilitate planning and policy development. The NDA classification codes are not indicative of the extent of innovation or therapeutic value that a drug represents. For details see MAPP 5018.2.
| Classification | Meaning |
|---|---|
| Type 1 | New molecular entity |
| Type 2 | New active ingredient |
| Type 3 | New dosage form |
| Type 4 | New combination |
| Type 5 | New formulation or other differences |
| Type 6 | New indication or claim, same applicant [no longer used] |
| Type 7 | Previously marketed but without an approved NDA |
| Type 8 | Rx to OTC |
| Type 9 | New indication or claim, drug not to be marketed under type 9 NDA after approval |
| Type 10 | New indication or claim, drug to be marketed under type 10 NDA after approval |
| Type 1/4 | Type 1, New molecular entity, and Type 4, New combination |
| Type 2/3 | Type 2, New active ingredient, and Type 3, New dosage form |
| Type 2/4 | Type 2, New active ingredient and Type 4, New combination |
| Type 3/4 | Type 3, New Dosage Form, and Type 4, New combination |
| Type 4/5 | Type 4 New Combination and Type 5 New Formulation or New Manufacturer |
Review designations for NDAs, BLAs, and efficacy supplements can be 'priority' or 'standard'.
- 'Priority' review designation is assigned to NDAs, BLAs, and efficacy supplements for drugs that treat serious conditions and provide significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions compared to available therapies.
- 'Standard' review designation is assigned to applications and efficacy supplements for drugs that do not meet the priority review designation criteria. For more information about the priority and standard designations, see MAPP 6020.3.
15. Can I get a copy of the Drugs@FDA database?
Yes. Please see 'Drugs@FDA Data Files' for information about the database tables and a link to the compressed file for downloading. The file does not include the scripts (programming) we use to produce the online version of Drugs@FDA. We provide this technical information for users who are familiar with working with databases or spreadsheets.
16. How can I get further assistance?
- For general drug information please contact FDA's Division of Drug Information (DDI).
- Phone: Toll Free (855) 543-3784 or (301) 796-3400
- Fax: (301) 431-6353
- Email: druginfo@fda.hhs.gov
- Mail:
Office of Communications
10001 New Hampshire Ave
Hillandale Building, Room 4147
Silver Spring, MD 20993
