Approved Risk Evaluation and Mitigation Strategies (REMS)
REMS Reports
- REMS count
Currently, there are 77 REMS.- 71 [92%] include "elements to assure safe use' (ETASU). REMS with ETASU typically require clinicians or health care settings to become certified prior to prescribing and to participate in additional REMS activities, such as training, patient counseling, and monitoring.
- 6 [8%] include only a "communication plan" REMS element which is informational in nature. These communication plans are typically composed of letters, websites, and fact sheets describing the specific safety risks identified in the REMS.
- [0%] include only the "medication guide" REMS element. Even products that do not have "medication guide" REMS elements may have medication guides as part of their labeling.
- [0%] include the "communication plan" AND "medication guide" REMS elements only.
- Released REMS
This report lists information for released REMS (products whose REMS program is no longer in effect) including the date the REMS was approved, date the REMS was released, and if the REMS is a shared system.
REMS Data Files and Historic REMS Information
The information presented on this website, as well as historic information about REMS and their modifications, is compiled in the REMS Data Files below. All files below include information about current REMS as well as REMS that are no longer in place.
-
Download REMS data (Includes Released REMS) (REMS.csv)
This file presents a list of all approved REMS, including REMS that are no longer in place. -
Download REMS Versions data (REMS_Versions.csv)
This file includes details on all modifications and revisions to each REMS program, including information on no-longer-current revisions and modifications. -
Download REMS Products data (REMS_Products.csv)
This file includes data on all of the drugs that have ever been part of a REMS program, including information on products that are no longer marketed and/or no longer subject to a REMS.
Data Description
The data available on this page is organized into four tables, each of which can be viewed on its own or in combination with other tables as part of a relational database. The entity-relationship diagram below shows the fields in each of these tables and how they should be linked together to form a comprehensive REMS database:
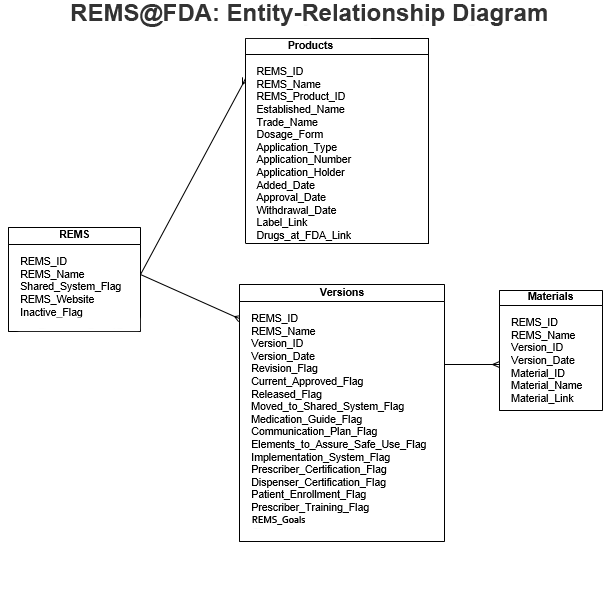
The following is a description of each table and its data:
- REMS
The REMS table includes one record for each REMS program, including REMS that are no longer in place. Detailed information about each REMS program is not included in this table, but can be found in the REMS Versions table.
This table includes the following fields:
- REMSID: A unique key used to identify each REMS.
- REMS_Name: The name used on the REMS website to refer to the REMS program. Generally, single-product REMS are referred to by the brand name of the product, while shared system REMS are referred to by the name of the molecule or class to which they apply.
- Shared_System_Flag: A flag that indicates whether a REMS is a shared system REMS.
- REMS_Website: A link to the application-holder's official website for the REMS.
- Inactive_Flag: A flag that indicates REMS programs that are no longer active. A REMS program may become inactive if the REMS requirement is released, if it is a single-product REMS that is incorporated into a shared system, or if all products under the REMS have been withdrawn and are published in the Federal Register (FR).
- Versions
The REMS Versions table includes a record for each change to the REMS, including a record for each newly approved REMS, an additional record for each modification or revision to that REMS, and a record when the REMS requirement is released or the REMS is moved to a shared system. For more information about REMS revisions and modifications, please see FDA's Draft Guidance, Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry.
This table includes the following fields.- REMSID: A unique key used to identify each REMS.
- REMS_Name: The name used on the REMS website to refer to the REMS program. Generally, single-product REMS are referred to by the brand name of the product, while shared system REMS are referred to by the name of the molecule or class to which they apply.
- VersionID: A unique key used to identify the version of the REMS.
- Version_Date: The date this version of the REMS was approved. The earliest version_date of the REMS is the date that the REMS was initially approved. For REMS that are no longer in place, the latest version_date indicates the date that the REMS was removed.
- Revision_Flag: A flag that indicates whether this version of the REMS is a revision as defined in FDA's Draft Guidance, Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry. REMS revisions are defined as being limited to editorial changes, corrections of typographical errors, and changes in the application holder name or address. If the flag is equal to 0, this indicates that this version of the REMS is a newly approved REMS or a modification to that REMS.
- Current_Approved_Flag: A flag that indicates whether this is the most recent version of a currently approved REMS (i.e., the version of the REMS that would appear on the REMS website homepage).
- Released_Flag: A flag that indicates whether the REMS was released as of that version_date.
- Moved_to_Shared_System_Flag: A flag that indicates whether the REMS was moved to a shared system as of that version_date.
- Medication_Guide_Flag: A flag that indicates whether a REMS has a Medication Guide as one of its elements. Note that many REMS products have medication guides that are not part of the REMS program and are not captured here. For a full list of medication guides, click here.
- Communication_Plan_Flag: A flag that indicates whether this version of the REMS has a communication plan as one of its elements.
- Elements_to_Assure_Safe_Use_Flag: A flag that indicates whether this version of the REMS has elements to assure safe use.
- Implementation_System_Flag: A flag that indicates whether this version of the REMS has an implementation system as one of its elements. This applies only to REMS with elements to assure safe use.
- Prescriber_Certification_Flag: A flag that indicates whether this version of the REMS requires prescribers to become certified in order to be able to prescribe the drug. This applies only to REMS with elements to assure safe use.
- Dispenser_Certification_Flag: A flag that indicates whether this version of the REMS requires dispenser to become certified in order to be able to dispense the drug. This applies only to REMS with elements to assure safe use.
- Patient_Enrollment_Flag: A flag that indicates whether this version of the REMS requires patients to be enrolled in the REMS program. This applies only to REMS with elements to assure safe use.
- Prescriber_Training_Flag: A flag that indicates whether the REMS provides training to prescriber as part of its Elements to Assure Safe Use.
- REMS_Goals: The goals for each REMS Program, as specified in the REMS Document.
- Products
The Products table includes a record for each application that has been subject to a REMS.
This table includes the following fields- REMSID: A unique key used to identify each REMS.
- REMS_Name: The name used on the REMS website to refer to the REMS program. Generally, single-product REMS are referred to by the brand name of the product, while shared system REMS are referred to by the name of the molecule or class to which they apply.
- ProductID: A unique key used to identify the drug
- Established_Name: The official nonproprietary name assigned to the drug. Generally, this is the "generic" name of the drug.
- Trade_Name: The proprietary or brand name for the drug, where it exists. Many generic products do not have trade names.
- Dosage_Form: The dosage form of the drug.
- Application_Type: The type of marketing approval the drug received. A drug may be marketed under a New Drug Application (NDA), Biologics License Application (BLA), or as a generic drug under an Abbreviated New Drug Application (ANDA).
- Application_Number: The number assigned by FDA staff to each application. One drug can have more than one application number if it has different dosage forms or routes of administration.
- Added_Date: The date the drug was added to the REMS. For most drugs, this is the same as the date that the REMS was initially put into place, but the dates may be different, if, for instance, a new drug is added to an existing shared system.
- Approval_Date: The date the drug was initially approved for marketing.
- Withdrawal_Date: The date published in the Federal Register announcing the drug was withdrawn from the market. If the drug was not withdrawn or the withdrawal was not announced in the Federal Register then this date will be blank.
- Label_Link: A link to the drug's label on DailyMed. DailyMed is a website hosted by the National Library of Medicine that provides information on the drug's current labeling. The current labeling shown on DailyMed may be different from the version that was initially approved by FDA and displayed at Drugs@FDA.
- Drugs_at_FDA_Link: A link to regulatory information about a drug at Drugs@FDA. Drugs@FDA is a catalog that provides information about FDA-approved products, including the product's approval history, FDA-approved labeling, therapeutically equivalent products, and consumer information.
*Many products within these REMS programs have Medication Guides not part of the REMS program. For a full list of Medication Guides click here.
