|
|
| [Code of Federal Regulations] |
| [Title 21, Volume 2] |
| [CITE: 21CFR101] |
TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUBCHAPTER B - FOOD FOR HUMAN CONSUMPTION
|
|
Subpart A - General Provisions
|
|
Sec. 101.1 Principal display panel of package form food.
|
|
The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label information required to be placed thereon by this part with clarity and conspicuousness and without obscuring design, vignettes, or crowding. Where packages bear alternate principal display panels, information required to be placed on the principal display panel shall be duplicated on each principal display panel. For the purpose of obtaining uniform type size in declaring the quantity of contents for all packages of substantially the same size, the term area of the principal display panel means the area of the side or surface that bears the principal display panel, which area shall be:
(a) In the case of a rectangular package where one entire side properly can be considered to be the principal display panel side, the product of the height times the width of that side;
(b) In the case of a cylindrical or nearly cylindrical container, 40 percent of the product of the height of the container times the circumference;
(c) In the case of any otherwise shaped container, 40 percent of the total surface of the container: Provided, however, That where such container presents an obvious "principal display panel" such as the top of a triangular or circular package of cheese, the area shall consist of the entire top surface. In determining the area of the principal display panel, exclude tops, bottoms, flanges at tops and bottoms of cans, and shoulders and necks of bottles or jars. In the case of cylindrical or nearly cylindrical containers, information required by this part to appear on the principal display panel shall appear within that 40 percent of the circumference which is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale.
|
|
|
Sec. 101.2 Information panel of package form food.
|
|
(a) The term information panel as it applies to packaged food means that part of the label immediately contiguous and to the right of the principal display panel as observed by an individual facing the principal display panel with the following exceptions:
(1) If the part of the label immediately contiguous and to the right of the principal display panel is too small to accommodate the necessary information or is otherwise unusable label space, e.g., folded flaps or can ends, the panel immediately contiguous and to the right of this part of the label may be used.
(2) If the package has one or more alternate principal display panels, the information panel is immediately contiguous and to the right of any principal display panel.
(3) If the top of the container is the principal display panel and the package has no alternate principal display panel, the information panel is any panel adjacent to the principal display panel.
(b) All information required to appear on the label of any package of food under §§ 101.4, 101.5, 101.8, 101.9, 101.13, 101.17, 101.36, subpart D of part 101, and part 105 of this chapter shall appear either on the principal display panel or on the information panel, unless otherwise specified by regulations in this chapter.
(c) All information appearing on the principal display panel or the information panel pursuant to this section shall appear prominently and conspicuously, but in no case may the letters and/or numbers be less than one-sixteenth inch in height unless an exemption pursuant to paragraph (f) of this section is established. The requirements for conspicuousness and legibility shall include the specifications of §§ 101.7(h)(1) and (2) and 101.15.
(1)(i) Soft drinks packaged in bottles manufactured before October 31, 1975 shall be exempt from the requirements prescribed by this section to the extent that information which is blown, lithographed, or formed onto the surface of the bottle is exempt from the size and placement requirements of this section.
(ii) Soft drinks packaged in bottles shall be exempt from the size and placement requirements prescribed by this section if all of the following conditions are met:
(A) If the soft drink is packaged in a bottle bearing a paper, plastic foam jacket, or foil label, or is packaged in a nonreusable bottle bearing a label lithographed onto the surface of the bottle or is packaged in metal cans, the product shall not be exempt from any requirement of this section other than the exemptions created by § 1.24(a)(5) (ii) and (v) of this chapter and the label shall bear all required information in the specified minimum type size, except the label will not be required to bear the information required by § 101.5 if this information appears on the bottle closure or on the lid of the can in a type size not less than one-sixteenth inch in height, or if embossed on the lid of the can in a type size not less than one-eighth inch in height.
(B) If the soft drink is packaged in a bottle which does not bear a paper, plastic foam jacket or foil label, or is packaged in a reusable bottle bearing a label lithographed onto the surface of the bottle:
(1 ) Neither the bottle nor the closure is required to bear nutrition labeling in compliance with § 101.9, except that any multiunit retail package in which it is contained shall bear nutrition labeling if required by § 101.9; and any vending machine in which it is contained shall bear nutrition labeling if nutrition labeling is not present on the bottle or closure, if required by § 101.9.
(2 ) All other information pursuant to this section shall appear on the top of the bottle closure prominently and conspicuously in letters and/or numbers no less than one thirty-second inch in height, except that if the information required by § 101.5 is placed on the side of the closure in accordance with § 1.24(a)(5)(ii) of this chapter, such information shall appear in letters and/or numbers no less than one-sixteenth inch in height.
(3 ) Upon the petition of any interested person demonstrating that the bottle closure is too small to accommodate this information, the Commissioner may by regulation establish an alternative method of disseminating such information. Information appearing on the closure shall appear in the following priority:
(i ) The statement of ingredients.
(ii ) The name and address of the manufacturer, packer, or distributor.
(iii ) The statement of identity.
(2) Individual serving-size packages of food served with meals in restaurants, institutions, and on board passenger carriers, and not intended for sale at retail, are exempt from type-size requirements of this paragraph, provided:
(i) The package has a total area of 3 square inches or less available to bear labeling;
(ii) There is insufficient area on the package available to print all required information in a type size of
1/16 inch in height;
(iii) The information required by paragraph (b) of this section appears on the label in accordance with the provisions of this paragraph, except that the type size is not less than
1/32 inch in height.
(d)(1) Except as provided by §§ 101.9(j)(13) and (j)(17) and 101.36(i)(2) and (i)(5), all information required to appear on the principal display panel or on the information panel under this section shall appear on the same panel unless there is insufficient space. In determining the sufficiency of the available space, except as provided by §§ 101.9(j)(17) and 101.36(i)(5), any vignettes, designs, and other nonmandatory label information shall not be considered. If there is insufficient space for all of this information to appear on a single panel, it may be divided between these two panels, except that the information required under any given section or part shall all appear on the same panel. A food whose label is required to bear the ingredient statement on the principal display panel may bear all other information specified in paragraph (b) of this section on the information panel.
(2) Any food, not otherwise exempted in this section, if packaged in a container consisting of a separate lid and body, and bearing nutrition labeling pursuant to § 101.9, and if the lid qualifies for and is designed to serve as a principal display panel, shall be exempt from the placement requirements of this section in the following respects:
(i) The name and place of business information required by § 101.5 shall not be required on the body of the container if this information appears on the lid in accordance with this section.
(ii) The nutrition information required by § 101.9 shall not be required on the lid if this information appears on the container body in accordance with this section.
(iii) The statement of ingredients required by § 101.4 shall not be required on the lid if this information appears on the container body in accordance with this section. Further, the statement of ingredients is not required on the container body if this information appears on the lid in accordance with this section.
(e) All information appearing on the information panel pursuant to this section shall appear in one place without other intervening material.
(f) If the label of any package of food is too small to accommodate all of the information required by §§ 101.4, 101.5, 101.8, 101.9, 101.13, 101.17, 101.36, subpart D of part 101, and part 105 of this chapter, the Commissioner may establish by regulation an acceptable alternative method of disseminating such information to the public, e.g., a type size smaller than one-sixteenth inch in height, or labeling attached to or inserted in the package or available at the point of purchase. A petition requesting such a regulation, as an amendment to this paragraph, shall be submitted under part 10 of this chapter.
[42 FR 14308, Mar. 15, 1977, as amended at 42 FR 15673, Mar. 22, 1977; 42 FR 45905, Sept. 13, 1977; 42 FR 47191, Sept. 20, 1977; 44 FR 16006, Mar. 16, 1979; 49 FR 13339, Apr. 4, 1984; 53 FR 16068, May 5, 1988; 58 FR 44030, Aug. 18, 1993; 60 FR 17205, Apr. 5, 1995; 62 FR 43074, Aug. 12, 1997; 62 FR 49847, Sept. 23, 1997; 63 FR 14817, Mar. 27, 1998; 81 FR 59131, Aug. 29, 2016]
|
|
|
Sec. 101.3 Identity labeling of food in packaged form.
|
|
(a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity.
(b) Such statement of identity shall be in terms of:
(1) The name now or hereafter specified in or required by any applicable Federal law or regulation; or, in the absence thereof,
(2) The common or usual name of the food; or, in the absence thereof,
(3) An appropriately descriptive term, or when the nature of the food is obvious, a fanciful name commonly used by the public for such food.
(c) Where a food is marketed in various optional forms (whole, slices, diced, etc.), the particular form shall be considered to be a necessary part of the statement of identity and shall be declared in letters of a type size bearing a reasonable relation to the size of the letters forming the other components of the statement of identity; except that if the optional form is visible through the container or is depicted by an appropriate vignette, the particular form need not be included in the statement. This specification does not affect the required declarations of identity under definitions and standards for foods promulgated pursuant to section 401 of the act.
(d) This statement of identity shall be presented in bold type on the principal display panel, shall be in a size reasonably related to the most prominent printed matter on such panel, and shall be in lines generally parallel to the base on which the package rests as it is designed to be displayed.
(e) Under the provisions of section 403(c) of the Federal Food, Drug, and Cosmetic Act, a food shall be deemed to be misbranded if it is an imitation of another food unless its label bears, in type of uniform size and prominence, the word "imitation" and, immediately thereafter, the name of the food imitated.
(1) A food shall be deemed to be an imitation and thus subject to the requirements of section 403(c) of the act if it is a substitute for and resembles another food but is nutritionally inferior to that food.
(2) A food that is a substitute for and resembles another food shall not be deemed to be an imitation provided it meets each of the following requirements:
(i) It is not nutritionally inferior to the food for which it substitutes and which it resembles.
(ii) Its label bears a common or usual name that complies with the provisions of § 102.5 of this chapter and that is not false or misleading, or in the absence of an existing common or usual name, an appropriately descriptive term that is not false or misleading. The label may, in addition, bear a fanciful name which is not false or misleading.
(3) A food for which a common or usual name is established by regulation (e.g., in a standard of identity pursuant to section 401 of the act, in a common or usual name regulation pursuant to part 102 of this chapter, or in a regulation establishing a nutritional quality guideline pursuant to part 104 of this chapter), and which complies with all of the applicable requirements of such regulation(s), shall not be deemed to be an imitation.
(4) Nutritional inferiority includes:
(i) Any reduction in the content of an essential nutrient that is present in a measurable amount, but does not include a reduction in the caloric or fat content provided the food is labeled pursuant to the provisions of § 101.9, and provided the labeling with respect to any reduction in caloric content complies with the provisions applicable to caloric content in part 105 of this chapter.
(ii) For the purpose of this section, a measurable amount of an essential nutrient in a food shall be considered to be 2 percent or more of the Daily Reference Value (DRV) of protein listed under § 101.9(c)(7)(iii) and of potassium listed under § 101.9(c)(9) per reference amount customarily consumed and 2 percent or more of the Reference Daily Intake (RDI) of any vitamin or mineral listed under § 101.9(c)(8)(iv) per reference amount customarily consumed, except that selenium, molybdenum, chromium, and chloride need not be considered.
(iii) If the Commissioner concludes that a food is a substitute for and resembles another food but is inferior to the food imitated for reasons other than those set forth in this paragraph, he may propose appropriate revisions to this regulation or he may propose a separate regulation governing the particular food.
(f) A label may be required to bear the percentage(s) of a characterizing ingredient(s) or information concerning the presence or absence of an ingredient(s) or the need to add an ingredient(s) as part of the common or usual name of the food pursuant to subpart B of part 102 of this chapter.
(g) Dietary supplements shall be identified by the term "dietary supplement" as a part of the statement of identity, except that the word "dietary" may be deleted and replaced by the name of the dietary ingredients in the product (e.g., calcium supplement) or an appropriately descriptive term indicating the type of dietary ingredients that are in the product (e.g., herbal supplement with vitamins).
[42 FR 14308, Mar. 15, 1977, as amended at 48 FR 10811, Mar. 15, 1983; 58 FR 2227, Jan. 6, 1993; 60 FR 67174, Dec. 28, 1995; 62 FR 49847, Sept. 23, 1997]
|
|
|
Sec. 101.4 Food; designation of ingredients.
|
|
(a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel in accordance with the provisions of § 101.2, except that ingredients in dietary supplements that are listed in the nutrition label in accordance with § 101.36 need not be repeated in the ingredient list. Paragraph (g) of this section describes the ingredient list on dietary supplement products.
(2) The descending order of predominance requirements of paragraph (a)(1) of this section do not apply to ingredients present in amounts of 2 percent or less by weight when a listing of these ingredients is placed at the end of the ingredient statement following an appropriate quantifying statement, e.g., "Contains __ percent or less of ______" or "Less than __ percent of ______." The blank percentage within the quantifying statement shall be filled in with a threshold level of 2 percent, or, if desired, 1.5 percent, 1.0 percent, or 0.5 percent, as appropriate. No ingredient to which the quantifying phrase applies may be present in an amount greater than the stated threshold.
(b) The name of an ingredient shall be a specific name and not a collective (generic) name, except that:
(1) Spices, flavorings, colorings and chemical preservatives shall be declared according to the provisions of § 101.22.
(2) An ingredient which itself contains two or more ingredients and which has an established common or usual name, conforms to a standard established pursuant to the Meat Inspection or Poultry Products Inspection Acts by the U.S. Department of Agriculture, or conforms to a definition and standard of identity established pursuant to section 401 of the Federal Food, Drug, and Cosmetic Act, shall be designated in the statement of ingredients on the label of such food by either of the following alternatives:
(i) By declaring the established common or usual name of the ingredient followed by a parenthetical listing of all ingredients contained therein in descending order of predominance except that, if the ingredient is a food subject to a definition and standard of identity established in subchapter B of this chapter that has specific labeling provisions for optional ingredients, optional ingredients may be declared within the parenthetical listing in accordance with those provisions.
(ii) By incorporating into the statement of ingredients in descending order of predominance in the finished food, the common or usual name of every component of the ingredient without listing the ingredient itself.
(3) Skim milk, concentrated skim milk, reconstituted skim milk, and nonfat dry milk may be declared as "skim milk" or "nonfat milk".
(4) Milk, concentrated milk, reconstituted milk, and dry whole milk may be declared as "milk".
(5) Bacterial cultures may be declared by the word "cultured" followed by the name of the substrate, e.g., "made from cultured skim milk or cultured buttermilk".
(6) Sweetcream buttermilk, concentrated sweetcream buttermilk, reconstituted sweetcream buttermilk, and dried sweetcream buttermilk may be declared as "buttermilk".
(7) Whey, concentrated whey, reconstituted whey, and dried whey may be declared as "whey".
(8) Cream, reconstituted cream, dried cream, and plastic cream (sometimes known as concentrated milk fat) may be declared as "cream".
(9) Butteroil and anhydrous butterfat may be declared as "butterfat".
(10) Dried whole eggs, frozen whole eggs, and liquid whole eggs may be declared as "eggs".
(11) Dried egg whites, frozen egg whites, and liquid egg whites may be declared as "egg whites".
(12) Dried egg yolks, frozen egg yolks, and liquid egg yolks may be declared as "egg yolks".
(13) [Reserved]
(14) Each individual fat and/or oil ingredient of a food intended for human consumption shall be declared by its specific common or usual name (e.g., "beef fat", "cottonseed oil") in its order of predominance in the food except that blends of fats and/or oils may be designated in their order of predominance in the foods as "______ shortening" or "blend of ______ oils", the blank to be filled in with the word "vegetable", "animal", "marine", with or without the terms "fat" or "oils", or combination of these, whichever is applicable if, immediately following the term, the common or usual name of each individual vegetable, animal, or marine fat or oil is given in parentheses, e.g., "vegetable oil shortening (soybean and cottonseed oil)". For products that are blends of fats and/or oils and for foods in which fats and/or oils constitute the predominant ingredient, i.e., in which the combined weight of all fat and/or oil ingredients equals or exceeds the weight of the most predominant ingredient that is not a fat or oil, the listing of the common or usual names of such fats and/or oils in parentheses shall be in descending order of predominance. In all other foods in which a blend of fats and/or oils is used as an ingredient, the listing of the common or usual names in parentheses need not be in descending order of predominance if the manufacturer, because of the use of varying mixtures, is unable to adhere to a constant pattern of fats and/or oils in the product. If the fat or oil is completely hydrogenated, the name shall include the term hydrogenated, or if partially hydrogenated, the name shall include the term partially hydrogenated. If each fat and/or oil in a blend or the blend is completely hydrogenated, the term "hydrogenated" may precede the term(s) describing the blend, e.g., "hydrogenated vegetable oil (soybean, cottonseed, and palm oils)", rather than preceding the name of each individual fat and/or oil; if the blend of fats and/or oils is partially hydrogenated, the term "partially hydrogenated" may be used in the same manner. Fat and/or oil ingredients not present in the product may be listed if they may sometimes be used in the product. Such ingredients shall be identified by words indicating that they may not be present, such as "or", "and/or", "contains one or more of the following:", e.g., "vegetable oil shortening (contains one or more of the following: cottonseed oil, palm oil, soybean oil)". No fat or oil ingredient shall be listed unless actually present if the fats and/or oils constitute the predominant ingredient of the product, as defined in this paragraph (b)(14).
(15) When all the ingredients of a wheat flour are declared in an ingredient statement, the principal ingredient of the flour shall be declared by the name(s) specified in §§ 137.105, 137.200, 137.220 and 137.225 of this chapter, i.e., the first ingredient designated in the ingredient list of flour, or bromated flour, or enriched flour, or self-rising flour is "flour", "white flour", "wheat flour", or "plain flour"; the first ingredient designated in the ingredient list of durum flour is "durum flour"; the first ingredient designated in the ingredient list of whole wheat flour, or bromated whole wheat flour is "whole wheat flour", "graham flour", or "entire wheat flour"; and the first ingredient designated in the ingredient list of whole durum wheat flour is "whole durum wheat flour".
(16) Ingredients that act as leavening agents in food may be declared in the ingredient statement by stating the specific common or usual name of each individual leavening agent in parentheses following the collective name "leavening", e.g., "leavening (baking soda, monocalcium phosphate, and calcium carbonate)". The listing of the common or usual name of each individual leavening agent in parentheses shall be in descending order of predominance: Except, That if the manufacturer is unable to adhere to a constant pattern of leavening agents in the product, the listing of individual leavening agents need not be in descending order of predominance. Leavening agents not present in the product may be listed if they are sometimes used in the product. Such ingredients shall be identified by words indicating that they may not be present, such as "or", "and/or", "contains one or more of the following:".
(17) Ingredients that act as yeast nutrients in foods may be declared in the ingredient statement by stating the specific common or usual name of each individual yeast nutrient in parentheses following the collective name "yeast nutrients", e.g., "yeast nutrients (calcium sulfate and ammonium phosphate)". The listing of the common or usual name of each individual yeast nutrient in parentheses shall be in descending order of predominance: Except, That if the manufacturer is unable to adhere to a constant pattern of yeast nutrients in the product, the listing of the common or usual names of individual yeast nutrients need not be in descending order of predominance. Yeast nutrients not present in the product may be listed if they are sometimes used in the product. Such ingredients shall be identified by words indicating that they may not be present, such as "or", "and/or", or "contains one or more of the following:".
(18) Ingredients that act as dough conditioners may be declared in the ingredient statement by stating the specific common or usual name of each individual dough conditioner in parentheses following the collective name "dough conditioner", e.g., "dough conditioners (L-cysteine, ammonium sulfate)". The listing of the common or usual name of each dough conditioner in parentheses shall be in descending order of predominance: Except, That if the manufacturer is unable to adhere to a constant pattern of dough conditioners in the product, the listing of the common or usual names of individual dough conditioners need not be in descending order of predominance. Dough conditioners not present in the product may be listed if they are sometimes used in the product. Such ingredients shall be identified by words indicating that they may not be present, such as "or", "and/or", or "contains one or more of the following:".
(19) Ingredients that act as firming agents in food (e.g., salts of calcium and other safe and suitable salts in canned vegetables) may be declared in the ingredient statement, in order of predominance appropriate for the total of all firming agents in the food, by stating the specific common or usual name of each individual firming agent in descending order of predominance in parentheses following the collective name "firming agents". If the manufacturer is unable to adhere to a constant pattern of firming agents in the food, the listing of the individual firming agents need not be in descending order of predominance. Firming agents not present in the product may be listed if they are sometimes used in the product. Such ingredients shall be identified by words indicating that they may not be present, such as "or", "and/or", "contains one or more of the following:".
(20) For purposes of ingredient labeling, the term sugar shall refer to sucrose, which is obtained from sugar cane or sugar beets in accordance with the provisions of § 184.1854 of this chapter.
(21) [Reserved]
(22) Wax and resin ingredients on fresh produce when such produce is held for retail sale, or when held for other than retail sale by packers or repackers shall be declared collectively by the phrase "coated with food-grade animal-based wax, to maintain freshness" or the phrase "coated with food-grade vegetable-, petroleum-, beeswax-, and/or shellac-based wax or resin, to maintain freshness" as appropriate. The terms "food-grade" and "to maintain freshness" are optional. The term lac-resin may be substituted for the term shellac.
(23) When processed seafood products contain fish protein ingredients consisting primarily of the myofibrillar protein fraction from one or more fish species and the manufacturer is unable to adhere to a constant pattern of fish species in the fish protein ingredient, because of seasonal or other limitations of species availability, the common or usual name of each individual fish species need not be listed in descending order of predominance. Fish species not present in the fish protein ingredient may be listed if they are sometimes used in the product. Such ingredients must be identified by words indicating that they may not be present, such as "or", "and/or", or "contains one or more of the following:" Fish protein ingredients may be declared in the ingredient statement by stating the specific common or usual name of each fish species that may be present in parentheses following the collective name "fish protein", e.g., "fish protein (contains one or more of the following: Pollock, cod, and/or pacific whiting)".
(c) When water is added to reconstitute, completely or partially, an ingredient permitted by paragraph (b) of this section to be declared by a class name, the position of the ingredient class name in the ingredient statement shall be determined by the weight of the unreconstituted ingredient plus the weight of the quantity of water added to reconstitute that ingredient, up to the amount of water needed to reconstitute the ingredient to single strength. Any water added in excess of the amount of water needed to reconstitute the ingredient to single strength shall be declared as "water" in the ingredient statement.
(d) When foods characterized on the label as "nondairy" contain a caseinate ingredient, the caseinate ingredient shall be followed by a parenthetical statement identifying its source. For example, if the manufacturer uses the term "nondairy" on a creamer that contains sodium caseinate, it shall include a parenthetical term such as "a milk derivative" after the listing of sodium caseinate in the ingredient list.
(e) If the percentage of an ingredient is included in the statement of ingredients, it shall be shown in parentheses following the name of the ingredient and expressed in terms of percent by weight. Percentage declarations shall be expressed to the nearest 1 percent, except that where ingredients are present at levels of 2 percent or less, they may be grouped together and expressed in accordance with the quantifying guidance set forth in paragraph (a)(2) of this section.
(f) Except as provided in § 101.100, ingredients that must be declared on labeling because there is no label for the food, including foods that comply with standards of identity, shall be listed prominently and conspicuously by common or usual name in the manner prescribed by paragraph (b) of this section.
(g) When present, the ingredient list on dietary supplement products shall be located immediately below the nutrition label, or, if there is insufficient space below the nutrition label, immediately contiguous and to the right of the nutrition label and shall be preceded by the word "Ingredients," unless some ingredients (i.e., sources) are identified within the nutrition label in accordance with § 101.36(d), in which case the ingredients listed outside the nutrition label shall be in a list preceded by the words "Other ingredients." Ingredients in dietary supplements that are not dietary ingredients or that do not contain dietary ingredients, such as excipients, fillers, artificial colors, artificial sweeteners, flavors, or binders, shall be included in the ingredient list.
(h) The common or usual name of ingredients of dietary supplements that are botanicals (including fungi and algae) shall be consistent with the names standardized in Herbs of Commerce, 1992 edition, which is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies may be obtained from the American Herbal Products Association, 8484 Georgia Ave., suite 370, Silver Spring, MD 20910, 301-588-1171, FAX 301-588-1174, e-mail: ahpa@ahpa.org, or may be examined at the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500, between 9 a.m. and 4 p.m., Monday through Friday, or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html. The listing of these names on the label shall be followed by statements of:
(1) The part of the plant (e.g., root, leaves) from which the dietary ingredient is derived (e.g., "Garlic bulb" or "Garlic (bulb)"), except that this designation is not required for algae. The name of the part of the plant shall be expressed in English (e.g., "flower" rather than "flos");
(2) The Latin binomial name of the plant, in parentheses, except that this name is not required when it is available in the reference entitled: Herbs of Commerce for the common or usual name listed on the label, and, when required, the Latin binomial name may be listed before the part of the plant. Any name in Latin form shall be in accordance with internationally accepted rules on nomenclature, such as those found in the International Code of Botanical Nomenclature and shall include the designation of the author or authors who published the Latin name, when a positive identification cannot be made in its absence. The International Code of Botanical Nomenclature (Tokyo Code), 1994 edition, a publication of the International Association for Plant Taxonomy, is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies of the International Code of Botanical Nomenclature may be obtained from Koeltz Scientific Books, D-61453 Konigstein, Germany, and University Bookstore, Southern Illinois University, Carbondale, IL 62901-4422, 618-536-3321, FAX 618-453-5207, or may be examined at the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500, between 9 a.m. and 4 p.m., Monday through Friday, or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
(3) On labels of single-ingredient dietary supplements that do not include an ingredient list, the identification of the Latin binomial name, when needed, and the part of the plant may be prominently placed on the principal display panel or information panel, or included in the nutrition label.
[42 FR 14308, Mar. 15, 1977, as amended at 43 FR 12858, Mar. 28, 1978; 43 FR 24519, June 6, 1978; 48 FR 8054, Feb. 25, 1983; 55 FR 17433, Apr. 25, 1990; 58 FR 2875, Jan. 6, 1993; 62 FR 49847, Sept. 23, 1997; 62 FR 64634, Dec. 8, 1997; 64 FR 50448, Sept. 17, 1999; 66 FR 17358, Mar. 30, 2001; 66 FR 66742, Dec. 27, 2001; 68 FR 15355, Mar. 31, 2003; 81 FR 5590, Feb. 3, 2016; 88 FR 17716, Mar. 24, 2023]
|
|
|
Sec. 101.5 Food; name and place of business of manufacturer, packer, or distributor.
|
|
(a) The label of a food in packaged form shall specify conspicuously the name and place of business of the manufacturer, packer, or distributor.
(b) The requirement for declaration of the name of the manufacturer, packer, or distributor shall be deemed to be satisfied, in the case of a corporation, only by the actual corporate name, which may be preceded or followed by the name of the particular division of the corporation. In the case of an individual, partnership, or association, the name under which the business is conducted shall be used.
(c) Where the food is not manufactured by the person whose name appears on the label, the name shall be qualified by a phrase that reveals the connection such person has with such food; such as "Manufactured for ______", "Distributed by ______", or any other wording that expresses the facts.
(d) The statement of the place of business shall include the street address, city, State, and ZIP code; however, the street address may be omitted if it is shown in a current city directory or telephone directory. The requirement for inclusion of the ZIP code shall apply only to consumer commodity labels developed or revised after the effective date of this section. In the case of nonconsumer packages, the ZIP code shall appear either on the label or the labeling (including invoice).
(e) If a person manufactures, packs, or distributes a food at a place other than his principal place of business, the label may state the principal place of business in lieu of the actual place where such food was manufactured or packed or is to be distributed, unless such statement would be misleading.
|
|
|
Sec. 101.7 Declaration of net quantity of contents.
|
|
(a) The principal display panel of a food in package form shall bear a declaration of the net quantity of contents. This shall be expressed in the terms of weight, measure, numerical count, or a combination of numerical count and weight or measure. The statement shall be in terms of fluid measure if the food is liquid, or in terms of weight if the food is solid, semisolid, or viscous, or a mixture of solid and liquid; except that such statement may be in terms of dry measure if the food is a fresh fruit, fresh vegetable, or other dry commodity that is customarily sold by dry measure. If there is a firmly established general consumer usage and trade custom of declaring the contents of a liquid by weight, or a solid, semisolid, or viscous product by fluid measure, it may be used. Whenever the Commissioner determines that an existing practice of declaring net quantity of contents by weight, measure, numerical count, or a combination in the case of a specific packaged food does not facilitate value comparisons by consumers and offers opportunity for consumer confusion, he will by regulation designate the appropriate term or terms to be used for such commodity.
(b)(1) Statements of weight shall be in terms of avoirdupois pound and ounce.
(2) Statements of fluid measure shall be in terms of the U.S. gallon of 231 cubic inches and quart, pint, and fluid ounce subdivisions thereof, and shall:
(i) In the case of frozen food that is sold and consumed in a frozen state, express the volume at the frozen temperature.
(ii) In the case of refrigerated food that is sold in the refrigerated state, express the volume at 40 deg.F (4 deg.C).
(iii) In the case of other foods, express the volume at 68 deg.F (20 deg.C).
(3) Statements of dry measure shall be in terms of the U.S. bushel of 2,150.42 cubic inches and peck, dry quart, and dry pint subdivisions thereof.
(c) When the declaration of quantity of contents by numerical count does not give adequate information as to the quantity of food in the package, it shall be combined with such statement of weight, measure, or size of the individual units of the foods as will provide such information.
(d) The declaration may contain common or decimal fractions. A common fraction shall be in terms of halves, quarters, eighths, sixteenths, or thirty-seconds; except that if there exists a firmly established general consumer usage and trade custom of employing different common fractions in the net quantity declaration of a particular commodity, they may be employed. A common fraction shall be reduced to its lowest terms; a decimal fraction shall not be carried out to more than two places. A statement that includes small fractions of an ounce shall be deemed to permit smaller variations than one which does not include such fractions.
(e) The declaration shall be located on the principal display panel of the label, and with respect to packages bearing alternate principal panels it shall be duplicated on each principal display panel.
(f) The declaration shall appear as a distinct item on the principal display panel, shall be separated (by at least a space equal to the height of the lettering used in the declaration) from other printed label information appearing above or below the declaration and (by at least a space equal to twice the width of the letter "N" of the style of type used in the quantity of contents statement) from other printed label information appearing to the left or right of the declaration. It shall not include any term qualifying a unit of weight, measure, or count (such as "jumbo quart" and "full gallon") that tends to exaggerate the amount of the food in the container. It shall be placed on the principal display panel within the bottom 30 percent of the area of the label panel in lines generally parallel to the base on which the package rests as it is designed to be displayed: Provided, That on packages having a principal display panel of 5 square inches or less, the requirement for placement within the bottom 30 percent of the area of the label panel shall not apply when the declaration of net quantity of contents meets the other requirements of this part.
(g) The declaration shall accurately reveal the quantity of food in the package exclusive of wrappers and other material packed therewith: Provided, That in the case of foods packed in containers designed to deliver the food under pressure, the declaration shall state the net quantity of the contents that will be expelled when the instructions for use as shown on the container are followed. The propellant is included in the net quantity declaration.
(h) The declaration shall appear in conspicuous and easily legible boldface print or type in distinct contrast (by typography, layout, color, embossing, or molding) to other matter on the package; except that a declaration of net quantity blown, embossed, or molded on a glass or plastic surface is permissible when all label information is so formed on the surface. Requirements of conspicuousness and legibility shall include the specifications that:
(1) The ratio of height to width (of the letter) shall not exceed a differential of 3 units to 1 unit (no more than 3 times as high as it is wide).
(2) Letter heights pertain to upper case or capital letters. When upper and lower case or all lower case letters are used, it is the lower case letter "o" or its equivalent that shall meet the minimum standards.
(3) When fractions are used, each component numeral shall meet one-half the minimum height standards.
(i) The declaration shall be in letters and numerals in a type size established in relationship to the area of the principal display panel of the package and shall be uniform for all packages of substantially the same size by complying with the following type specifications:
(1) Not less than one-sixteenth inch in height on packages the principal display panel of which has an area of 5 square inches or less.
(2) Not less than one-eighth inch in height on packages the principal display panel of which has an area of more than 5 but not more than 25 square inches.
(3) Not less than three-sixteenths inch in height on packages the principal display panel of which has an area of more than 25 but not more than 100 square inches.
(4) Not less than one-fourth inch in height on packages the principal display panel of which has an area of more than 100 square inches, except not less than
1/2 inch in height if the area is more than 400 square inches.
Where the declaration is blown, embossed, or molded on a glass or plastic surface rather than by printing, typing, or coloring, the lettering sizes specified in paragraphs (h)(1) through (4) of this section shall be increased by one-sixteenth of an inch.
(j) On packages containing less than 4 pounds or 1 gallon and labeled in terms of weight or fluid measure:
(1) The declaration shall be expressed both in ounces, with identification by weight or by liquid measure and, if applicable (1 pound or 1 pint or more) followed in parentheses by a declaration in pounds for weight units, with any remainder in terms of ounces or common or decimal fractions of the pound (see examples set forth in paragraphs (m) (1) and (2) of this section), or in the case of liquid measure, in the largest whole units (quarts, quarts and pints, or pints, as appropriate) with any remainder in terms of fluid ounces or common or decimal fractions of the pint or quart (see examples in paragraphs (m) (3) and (4) of this section).
(2) If the net quantity of contents declaration appears on a random package, that is a package which is one of a lot, shipment, or delivery of packages of the same consumer commodity with varying weights and with no fixed weight pattern, it may, when the net weight exceeds 1 pound, be expressed in terms of pounds and decimal fractions of the pound carried out to not more than two decimal places. When the net weight does not exceed 1 pound, the declaration on the random package may be in decimal fractions of the pound in lieu of ounces (see example in paragraph (m)(5) of this section).
(3) The declaration may appear in more than one line. The term "net weight" shall be used when stating the net quantity of contents in terms of weight. Use of the terms "net" or "net contents" in terms of fluid measure or numerical count is optional. It is sufficient to distinguish avoirdupois ounce from fluid ounce through association of terms; for example, "Net wt. 6 oz" or "6 oz Net wt." and "6 fl oz" or "Net contents 6 fl oz".
(k) On packages containing 4 pounds or 1 gallon or more and labeled in terms of weight or fluid measure, the declaration shall be expressed in pounds for weight units with any remainder in terms of ounces or common or decimal fraction of the pound, or in the case of fluid measure, it shall be expressed in the largest whole unit (gallons followed by common or decimal fraction of a gallon or by the next smaller whole unit or units (quarts, or quarts and pints)) with any remainder in terms of fluid ounces or common or decimal fractions of the pint or quart (see paragraph (m)(6) of this section).
(l) [Reserved]
(m) Examples:
(1) A declaration of 1
1/2 pounds weight shall be expressed as "Net Wt. 24 oz (1 lb 8 oz)," "Net Wt. 24 oz (1
1/2 lb)," or "Net Wt. 24 oz (1.5 lb)".
(2) A declaration of three-fourths pound avoirdupois weight shall be expressed as "Net Wt. 12 oz".
(3) A declaration of 1 quart liquid measure shall be expressed as "Net 32 fl oz (1 qt)".
(4) A declaration of 1
3/4 quarts liquid measure shall be expressed as "Net contents 56 fluid ounces (1 quart 1
1/2 pints)" or as "Net 56 fluid oz (1 qt 1 pt 8 oz)", but not in terms of quart and ounce such as "Net 56 fluid oz (1 quart 24 ounces)".
(5) On a random package, declaration of three-fourths pound avoirdupois may be expressed as "Net Wt. .75 lb".
(6) A declaration of 2
1/2 gallons liquid measure shall be expressed as "Net contents 2
1/2 gallons," "Net contents 2.5 gallons," or "Net contents 2 gallons 2 quarts" and not as "2 gallons 4 pints".
(n) For quantities, the following abbreviations and none other may be employed (periods and plural forms are optional):
(o) Nothing in this section shall prohibit supplemental statements at locations other than the principal display panel(s) describing in nondeceptive terms the net quantity of contents; Provided, that such supplemental statements of net quantity of contents shall not include any term qualifying a unit of weight, measure, or count that tends to exaggerate the amount of the food contained in the package; for example, "jumbo quart" and "full gallon". Dual or combination declarations of net quantity of contents as provided for in paragraphs (a), (c), and (j) of this section (for example, a combination of net weight plus numerical count, net contents plus dilution directions of a concentrate, etc.) are not regarded as supplemental net quantity statements and may be located on the principal display panel.
(p) A separate statement of the net quantity of contents in terms of the metric system is not regarded as a supplemental statement and an accurate statement of the net quantity of contents in terms of the metric system of weight or measure may also appear on the principal display panel or on other panels.
(q) The declaration of net quantity of contents shall express an accurate statement of the quantity of contents of the package. Reasonable variations caused by loss or gain of moisture during the course of good distribution practice or by unavoidable deviations in good manufacturing practice will be recognized. Variations from stated quantity of contents shall not be unreasonably large.
(r) The declaration of net quantity of contents on pickles and pickle products, including relishes but excluding one or two whole pickles in clear plastic bags which may be declared by count, shall be expressed in terms of the U.S. gallon of 231 cubic inches and quart, pint, and fluid ounce subdivisions thereof.
(s) On a multiunit retail package, a statement of the quantity of contents shall appear on the outside of the package and shall include the number of individual units, the quantity of each individual unit, and, in parentheses, the total quantity of contents of the multiunit package in terms of avoirdupois or fluid ounces, except that such declaration of total quantity need not be followed by an additional parenthetical declaration in terms of the largest whole units and subdivisions thereof, as required by paragraph (j)(1) of this section. A multiunit retail package may thus be properly labeled: "6-16 oz bottles - (96 fl oz)" or "3-16 oz cans - (net wt. 48 oz)". For the purposes of this section, "multiunit retail package" means a package containing two or more individually packaged units of the identical commodity and in the same quantity, intended to be sold as part of the multiunit retail package but capable of being individually sold in full compliance with all requirements of the regulations in this part. Open multiunit retail packages that do not obscure the number of units or prevent examination of the labeling on each of the individual units are not subject to this paragraph if the labeling of each individual unit complies with the requirements of paragraphs (f) and (i) of this section. The provisions of this section do not apply to that butter or margarine covered by the exemptions in § 1.24(a) (10) and (11) of this chapter.
(t) Where the declaration of net quantity of contents is in terms of net weight and/or drained weight or volume and does not accurately reflect the actual quantity of the contents or the product falls below the applicable standard of fill of container because of equipment malfunction or otherwise unintentional product variation, and the label conforms in all other respects to the requirements of this chapter (except the requirement that food falling below the applicable standard of fill of container shall bear the general statement of substandard fill specified in § 130.14(b) of this chapter), the mislabeled food product, including any food product that fails to bear the general statement of substandard fill specified in § 130.14(b) of this chapter, may be sold by the manufacturer or processor directly to institutions operated by Federal, State or local governments (schools, prisons, hospitals, etc.): Provided, That:
(1) The purchaser shall sign a statement at the time of sale stating that he is aware that the product is mislabeled to include acknowledgment of the nature and extent of the mislabeling, (e.g., "Actual net weight may be as low as __% below labeled quantity") and that any subsequent distribution by him of said product except for his own institutional use is unlawful. This statement shall be kept on file at the principal place of business of the manufacturer or processor for 2 years subsequent to the date of shipment of the product and shall be available to the Food and Drug Administration upon request.
(2) The product shall be labeled on the outside of its shipping container with the statement(s):
(i) When the variation concerns net weight and/or drained weight or volume, "Product Mislabeled. Actual net weight (drained weight or volume where appropriate) may be as low as __% below labeled quantity. This Product Not for Retail Distribution", the blank to be filled in with the maximum percentage variance between the labeled and actual weight or volume of contents of the individual packages in the shipping container, and
(ii) When the variation is in regard to a fill of container standard, "Product Mislabeled. Actual fill may be as low as __% below standard of fill. This Product Not for Retail Distribution".
(3) The statements required by paragraphs (t)(2) (i) and (ii) of this section, which may be consolidated where appropriate, shall appear prominently and conspicuously as compared to other printed matter on the shipping container and in boldface print or type on a clear, contrasting background in order to render them likely to be read and understood by the purchaser under ordinary conditions of purchase.
weight wt ounce oz pound lb gallon gal pint pt quart qt fluid fl [42 FR 14308, Mar. 15, 1977, as amended at 42 FR 15673, Mar. 22, 1977. Redesignated at 81 FR 59131, Aug. 29, 2016]
|
|
|
Sec. 101.8 Vending machines.
|
|
(a) Definitions. The definitions of terms in section 201 of the Federal Food, Drug, and Cosmetic Act apply to such terms when used in this section. In addition, for the purposes of this section:
Authorized official of a vending machine operator means an owner, operator, agent in charge, or any other person authorized by a vending machine operator who is not otherwise subject to section 403(q)(5)(H)(viii) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 343(q)(5)(H)(viii)), to register the vending machine operator with the Food and Drug Administration ("FDA") for purposes of paragraph (d) of this section.
Vending machine means a self-service machine that, upon insertion of a coin, paper currency, token, card, or key, or by optional manual operation, dispenses servings of food in bulk or in packages, or prepared by the machine, without the necessity of replenishing the machine between each vending operation.
Vending machine operator means a person(s) or entity that controls or directs the function of the vending machine, including deciding which articles of food are sold from the machine or the placement of the articles of food within the vending machine, and is compensated for the control or direction of the function of the vending machine.
(b) Articles of food not covered. Articles of food sold from a vending machine are not covered vending machine food if:
(1) The prospective purchaser can view:
(i) The calories, serving size, and servings per container listed in the Nutrition Facts label on the vending machine food without any obstruction. The Nutrition Facts label must be in the format required in § 101.9(c) and (d). The Nutrition Facts label must be in a size that permits the prospective purchaser to be able to easily read the nutrition information contained in the Nutrition Facts label on the article of food in the vending machine. Smaller formats allowed for Nutrition Facts for certain food labeling under FDA regulation at § 101.9 are not considered to be a size that a prospective purchaser is able to easily read; or
(ii) The calories, serving size, and servings per container listed in a reproduction of the Nutrition Facts label on the vending machine food, provided that the reproduction is a reproduction of an actual Nutrition Facts label that complies with § 101.9 for a vending machine food, is presented in a size that permits the prospective purchaser to be able to easily read the nutrition information, and the calories, serving size, and servings per container are displayed by the vending machine before the prospective purchaser makes his or her purchase; or
(2) The prospective purchaser can otherwise view visible nutrition information, including, at a minimum, the total number of calories for the article of food as sold at the point of purchase. This visible nutrition information must appear on the food label itself. The visible nutrition information must be clear and conspicuous and able to be easily read on the article of food while in the vending machine, in a type size at least 150 percent of the size required by § 101.7(i) for the net quantity of contents declaration on the front of the package, and with sufficient color and contrasting background to other print on the label to permit the prospective purchaser to clearly distinguish the information.
(c) Requirements for calorie labeling for certain food sold from vending machines - (1) Applicability; covered vending machine food. For the purposes of this section, the term "covered vending machine food" means an article of food that is:
(i) Sold from a vending machine that does not permit the prospective purchaser to examine the Nutrition Facts label prior to purchase as provided in paragraph (b)(1) of this section or otherwise provide visible nutrition information at the point of purchase as provided in paragraph (b)(2) of this section; and
(ii) Sold from a vending machine that:
(A) Is operated by a person engaged in the business of owning or operating 20 or more vending machines; or
(B) Is operated by a vending machine operator that has voluntarily elected to be subject to the requirements of this section by registering with FDA under paragraph (d) of this section.
(2) Calorie declaration. (i) The number of calories for a covered vending machine food must be declared in the following manner:
(A) To the nearest 5-calorie increment up to and including 50 calories and 10-calorie increment above 50 calories, except that amounts less than 5 calories may be expressed as zero.
(B) The term "Calories" or "Cal" must appear adjacent to the caloric content value for each food in the vending machine.
(C) The calorie declaration for a packaged food must include the total calories present in the packaged food, regardless of whether the packaged food contains a single serving or multiple servings. The vending machine operator may voluntarily disclose calories per serving in addition to the total calories for the food.
(D) If a covered vending machine food is one where the prospective purchaser selects among options to produce a final vended product (e.g., vended coffee, hot chocolate or tea with options for added sugar, sugar substitute, milk, and cream), calories must be declared per option or for the final vended products.
(ii) Calorie declarations for covered vending machine food must be clear and conspicuous and placed prominently in the following manner:
(A) The calorie declarations may be placed on a sign in close proximity to the article of food or selection button, i.e., in, on, or adjacent to the vending machine, but not necessarily attached to the vending machine, so long as the calorie declaration is visible at the same time as the food, its name, price, selection button, or selection number is visible. The sign must give calorie declarations for those articles of food that are sold from that particular vending machine.
(B) When the calorie declaration is in or on the vending machine, the calorie declaration must be in a type size no smaller than the name of the food on the machine (not the label), selection number, or price of the food as displayed on the vending machine, whichever is smallest, with the same prominence, i.e., the same color, or in a color at least as conspicuous, as the color of the name, if applicable, or price of the food or selection number, and the same contrasting background, or a background at least as contrasting as the background used for the item it is in closest proximity to, i.e., name, selection number, or price of the food item as displayed on the machine.
(C) When the calorie declaration is on a sign adjacent to the vending machine, the calorie declaration must be in a type size large enough to render it likely to be read and understood by the prospective purchaser under customary conditions of purchase and use, and in a type that is all black or one color on a white or other neutral background that contrasts with the type color.
(D) Where the vending machine only displays a picture or other representation or name of the food item, the calorie declaration must be in close proximity to the picture or other representation or name, or in close proximity to the selection button.
(E) For electronic vending machines (e.g., machines with digital or electronic or liquid crystal display (LCD) displays), the calorie declaration must be displayed before the prospective purchaser makes his or her purchase.
(F) For vending machines with few choices, e.g., popcorn, the calorie declaration may appear on the face of the machine so long as the declaration is prominent, not crowded by other labeling on the machine, and the type size is no smaller than the name of the food on the machine (not the label), selection number, or price of the food as displayed on the vending machine, whichever is smallest.
(d) Voluntary provision of calorie labeling for foods sold from vending machines - (1) Applicability. A vending machine operator that is not subject to the requirements of section 403(q)(5)(H)(viii) of the Federal Food, Drug, and Cosmetic Act may, through its authorized official, voluntarily register with FDA to be subject to the requirements established in paragraph (c)(2) of this section. An authorized official of a vending machine operator that voluntarily registers cannot be subject to any State or local nutrition labeling requirements that are not identical to the requirements in 403(q)(5)(H) of the Federal Food, Drug, and Cosmetic Act.
(2) Who may register ? A vending machine operator that is not otherwise subject to the requirements of section 403(q)(5)(H) of the Federal Food, Drug, and Cosmetic Act may register with FDA.
(3) What information is required ? The vending machine operator must provide FDA with the following information:
(i) The contact information (including name, address, phone number, email address), for the vending machine operator;
(ii) The address of the location of each vending machine owned or operated by the vending machine operator that is being registered;
(iii) Preferred mailing address (if different from the vending machine operator address), for purposes of receiving correspondence; and
(iv) Certification that the information submitted is true and accurate, that the person or firm submitting it is authorized to do so, and that each registered vending machine will be subject to the requirements of this section.
(v) Information should be submitted by email by typing complete information into the portable document format (PDF) form, saving it on the registrant's computer, and sending it by email to menulawregistration@fda.hhs.gov. If email is not available, the registrant can either fill in the PDF form and print it out (or print out the blank PDF and fill in the information by hand or typewriter), and either fax the completed form to 301-436-2804 or mail it to FDA, CFSAN Menu and Vending Machine Labeling Registration, White Oak Building 22, rm. 0209, 10903 New Hampshire Ave., Silver Spring, MD 20993.
(vi) Authorized officials of a vending machine operator who elect to be subject to the Federal requirements can register by visiting http://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm217762.htm. FDA has created a form that contains fields requesting the information in paragraph (d) of this section and made the form available at this Web site. Registrants must use this form to ensure that complete information is submitted.
(vii) To keep the establishment's registration active, the authorized official of the vending machine operator must register every other year within 60 days prior to the expiration of the vending machine operator's current registration with FDA. Registration will automatically expire if not renewed.
(e) Vending machine operator contact information. (1) A vending machine operator that is subject to section 403(q)(5)(H)(viii) of the Federal Food, Drug, and Cosmetic Act or a vending machine operator that voluntarily registers to be subject to the requirements under paragraph (d) of this section must provide its contact information for vending machines selling covered vending machine food. The contact information must list the vending machine operator's name, telephone number, and mailing address or email address.
(2) The contact information must be readable and may be placed on the face of the vending machine, or otherwise must be placed with the calorie declarations as described in paragraph (c)(2)(ii) of this section (i.e., on the sign in, on, or adjacent to the vending machine).
(f) Signatures. Signatures obtained under paragraph (d) of this section that meet the definition of electronic signatures in § 11.3(b)(7) of this chapter are exempt from the requirements of part 11 of this chapter.
[79 FR 71291, Dec. 1, 2014, as amended at 84 FR 57610, Oct. 28, 2019]
|
|
|
Sec. 101.9 Nutrition labeling of food.
|
|
(a) Nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is provided for the product in paragraph (j) of this section.
(1) When food is in package form, the required nutrition labeling information shall appear on the label in the format specified in this section.
(2) When food is not in package form, the required nutrition labeling information shall be displayed clearly at the point of purchase (e.g., on a counter card, sign, tag affixed to the product, or some other appropriate device). Alternatively, the required information may be placed in a booklet, looseleaf binder, or other appropriate format that is available at the point of purchase.
(3) Solicitation of requests for nutrition information by a statement "For nutrition information write to ______________________ " on the label or in the labeling or advertising for a food, or providing such information in a direct written reply to a solicited or unsolicited request, does not subject the label or the labeling of a food exempted under paragraph (j) of this section to the requirements of this section if the reply to the request conforms to the requirements of this section.
(4) If any vitamin or mineral is added to a food so that a single serving provides 50 percent or more of the Reference Daily Intake (RDI) for the age group for which the product is intended, as specified in paragraph (c)(8)(iv) of this section, of any one of the added vitamins or minerals, unless such addition is permitted or required in other regulations, e.g., a standard of identity or nutritional quality guideline, or is otherwise exempted by the Commissioner, the food shall be considered a food for special dietary use within the meaning of § 105.3(a)(1)(iii) of this chapter.
(b) Except as provided in § 101.9(h)(3), all nutrient and food component quantities shall be declared in relation to a serving as defined in this section.
(1) The term serving or serving size means an amount of food customarily consumed per eating occasion by persons 4 years of age or older which is expressed in a common household measure that is appropriate to the food. When the food is specially formulated or processed for use by infants or by toddlers, a serving or serving size means an amount of food customarily consumed per eating occasion by infants up to 12 months of age or by children 1 through 3 years of age, respectively.
(2) Except as provided in paragraphs (b)(3), (b)(4), and (b)(6) of this section and for products that are intended for weight control and are available only through a weight-control or weight-maintenance program, serving size declared on a product label shall be determined from the "Reference Amounts Customarily Consumed Per Eating Occasion * * * *" (reference amounts) that appear in § 101.12(b) using the procedures described below. For products that are both intended for weight control and available only through a weight-control program, a manufacturer may determine the serving size that is consistent with the meal plan of the program. Such products must bear a statement, "for sale only through the ______ program" (fill in the blank with the name of the appropriate weight-control program, e.g., Smith's Weight Control), on the principal display panel. However, the reference amounts in § 101.12(b) shall be used for purposes of evaluating whether weight-control products that are available only through a weight-control program qualify for nutrient content claims or health claims.
(i) For products in discrete units (e.g., muffins, sliced products, such as sliced bread, or individually packaged products within a multiserving package) and for products which consist of two or more foods packaged and presented to be consumed together where the ingredient represented as the main ingredient is in discrete units (e.g., pancakes and syrup), the serving size shall be declared as follows:
(A) If a unit weighs 50 percent or less of the reference amount, the serving size shall be the number of whole units that most closely approximates the reference amount for the product category;
(B) If a unit weighs more than 50 percent, but less than 67 percent of the reference amount, the manufacturer may declare one unit or two units as the serving size;
(C) If a unit weighs 67 percent or more, but less than 200 percent of the reference amount, the serving size shall be one unit;
(D) If a unit weighs at least 200 percent and up to and including 300 percent of the applicable reference amount, the serving size shall be the amount that approximates the reference amount. In addition to providing a column within the Nutrition Facts label that lists the quantitative amounts and percent Daily Values per serving size, the manufacturer shall provide a column within the Nutrition Facts label that lists the quantitative amounts and percent Daily Values per individual unit. The first column would be based on the serving size for the product and the second column would be based on the individual unit. The exemptions in paragraphs (b)(12)(i)(A), (B), and (C) of this section apply to this provision.
(E) The serving size for maraschino cherries shall be expressed as 1 cherry with the parenthetical metric measure equal to the average weight of a medium size cherry.
(F) The serving size for products that naturally vary in size (e.g., pickles, shellfish, whole fish, and fillet of fish) may be the amount in ounces that most closely approximates the reference amount for the product category. Manufacturers shall adhere to the requirements in paragraph (b)(5)(vi) of this section for expressing the serving size in ounces.
(G) For products which consist of two or more foods packaged and presented to be consumed together where the ingredient represented as the main ingredient is in discrete units (e.g., pancakes and syrup), the serving size may be the number of discrete units represented as the main ingredient plus proportioned minor ingredients used to make the reference amount for the combined product determined in § 101.12(f).
(H) For packages containing several individual single-serving containers, each of which is labeled with all required information including nutrition labeling as specified in § 101.9 (that is, are labeled appropriately for individual sale as single-serving containers), the serving size shall be 1 unit.
(ii) For products in large discrete units that are usually divided for consumption (e.g., cake, pie, pizza, melon, cabbage), for unprepared products where the entire contents of the package is used to prepare large discrete units that are usually divided for consumption (e.g., cake mix, pizza kit), and for products which consist of two or more foods packaged and presented to be consumed together where the ingredient represented as the main ingredient is a large discrete unit usually divided for consumption (e.g., prepared cake packaged with a can of frosting), the serving size shall be the fractional slice of the ready-to-eat product (e.g.,
1/12 cake,
1/8 pie,
1/4 pizza,
1/4 melon,
1/6 cabbage) that most closely approximates the reference amount for the product category, and may be the fraction of the package used to make the reference amount for the unprepared product determined in § 101.12(c) or the fraction of the large discrete unit represented as the main ingredient plus proportioned minor ingredients used to make the reference amount for the combined product determined in § 101.12(f). In expressing the fractional slice, manufacturers shall use
1/2,
1/3,
1/4,
1/5,
1/6, or smaller fractions that can be generated by further division by 2 or 3.
(iii) For nondiscrete bulk products (e.g., breakfast cereal, flour, sugar, dry mixes, concentrates, pancake mixes, macaroni and cheese kits), and for products which consist of two or more foods packaged and presented to be consumed together where the ingredient represented as the main ingredient is a bulk product (e.g., peanut butter and jelly), the serving size shall be the amount in household measure that most closely approximates the reference amount for the product category and may be the amount of the bulk product represented as the main ingredient plus proportioned minor ingredients used to make the reference amount for the combined product determined in § 101.12(f).
(3) The serving size for meal products and main dish products as defined in § 101.13 (l) and (m) that comes in single-serving containers as defined in paragraph (b)(6) of this section shall be the entire content (edible portion only) of the package. Serving size for meal products and main dish products in multiserving containers shall be based on the reference amount applicable to the product in § 101.12(b) if the product is listed in § 101.12(b). Serving size for meal products and main dish products in multiserving containers that are not listed in § 101.12(b) shall be based on the reference amount according to § 101.12(f).
(4) A variety pack, such as a package containing several varieties of single-serving units as defined in paragraph (b)(2)(i) of this section, and a product having two or more compartments with each compartment containing a different food, shall provide nutrition information for each variety or food per serving size that is derived from the reference amount in § 101.12(b) applicable for each variety or food and the procedures to convert the reference amount to serving size in paragraph (b)(2) of this section.
(5) For labeling purposes, the term common household measure or common household unit means cup, tablespoon, teaspoon, piece, slice, fraction (e.g.,
1/4 pizza), ounce (oz), fluid ounce (fl oz), or other common household equipment used to package food products (e.g., jar, tray). In expressing serving size in household measures, except as specified in paragraphs (b)(5)(iv), (b)(5)(v), (b)(5)(vi), and (b)(5)(vii) of this section, the following rules shall be used:
(i) Cups, tablespoons, or teaspoons shall be used wherever possible and appropriate except for beverages. For beverages, a manufacturer may use fluid ounces. Cups shall be expressed in 1/4- or 1/3-cup increments. Tablespoons shall be expressed as 1, 1 1/3, 1 1/2, 1 2/3, 2, or 3 tablespoons. Teaspoons shall be expressed as 1/8, 1/4, 1/2, 3/4, 1, or 2 teaspoons.
(ii) If cups, tablespoons or teaspoons are not applicable, units such as piece, slice, tray, jar, and fraction shall be used.
(iii) If paragraphs (b)(5)(i) and (b)(5)(ii) of this section are not applicable, ounces may be used with an appropriate visual unit of measure such as a dimension of a piece, e.g., 1 oz (28 g/about
1/2 pickle). Ounce measurements shall be expressed in 0.5 oz increments most closely approximating the reference amount.
(iv) A description of the individual container or package shall be used for single serving containers and for individually packaged products within multiserving containers (e.g., can, box, package). A description of the individual unit shall be used for other products in discrete units (e.g., piece, slice, cracker, bar).
(v) For unprepared products where the entire contents of the package is used to prepare large discrete units that are usually divided for consumption (e.g., cake mix, pizza kit), the fraction or portion of the package may be used.
(vi) Ounces with an appropriate visual unit of measure, as described in paragraph (b)(5)(iii) of this section, may be used for products that naturally vary in size as provided for in paragraph (b)(2)(i)(F) of this section.
(vii) As provided for in § 101.9(h)(1), for products that consist of two or more distinct ingredients or components packaged and presented to be consumed together (e.g. dry macaroni and cheese mix, cake and muffin mixes with separate ingredient packages, pancakes and syrup), nutrition information may be declared for each component or as a composite. The serving size may be provided in accordance with the provisions of paragraphs (b)(2)(i), (b)(2)(ii), and (b)(2)(iii) of this section, or alternatively in ounces with an appropriate visual unit of measure, as described in paragraph (b)(5)(iii) of this section (e.g., declared as separate components: "3 oz dry macaroni (84 g/about
2/3 cup)" and "1 oz dry cheese mix (28 g/about 2 tbsp);" declared as a composite value: "4 oz (112 g/about
2/3 cup macaroni and 2 tbsp dry cheese mix)").
(viii) For nutrition labeling purposes, a teaspoon means 5 milliliters (mL), a tablespoon means 15 mL, a cup means 240 mL, 1 fl oz means 30 mL, and 1 oz in weight means 28 g.
(ix) When a serving size, determined from the reference amount in § 101.12(b) and the procedures described in this section, falls exactly half way between two serving sizes, e.g., 2.5 tbsp, manufacturers shall round the serving size up to the next incremental size.
(6) A product that is packaged and sold individually that contains less than 200 percent of the applicable reference amount must be considered to be a single-serving container, and the entire content of the product must be labeled as one serving. In addition to providing a column within the Nutrition Facts label that lists the quantitative amounts and percent Daily Values per serving, for a product that is packaged and sold individually that contains more than 150 percent and less than 200 percent of the applicable reference amount, the Nutrition Facts label may voluntarily provide, to the left of the column that provides nutrition information per container (i.e., per serving), an additional column that lists the quantitative amounts and percent Daily Values per common household measure that most closely approximates the reference amount.
(7) A label statement regarding a serving shall be the serving size expressed in common household measures as set forth in paragraphs (b)(2) through (b)(6) of this section and shall be followed by the equivalent metric quantity in parenthesis (fluids in milliliters and all other foods in grams) except for single-serving containers.
(i) For a single-serving container, the parenthetical metric quantity, which will be presented as part of the net weight statement on the principal display panel, is not required except where nutrition information is required on a drained weight basis according to § 101.9(b)(9). However, if a manufacturer voluntarily provides the metric quantity on products that can be sold as single servings, then the numerical value provided as part of the serving size declaration must be identical to the metric quantity declaration provided as part of the net quantity of contents statement.
(ii) The gram or milliliter quantity equivalent to the household measure should be rounded to the nearest whole number except for quantities that are less than 5 g (mL). The gram (mL) quantity between 2 and 5 g (mL) should be rounded to the nearest 0.5 g (mL) and the g (mL) quantity less than 2 g (mL) should be expressed in 0.1-g (mL) increments.
(iii) In addition, serving size may be declared in ounce and fluid ounce, in parenthesis, following the metric measure separated by a slash where other common household measures are used as the primary unit for serving size, e.g., 1 slice (28 g/1 oz) for sliced bread. The ounce quantity equivalent to the metric quantity should be expressed in 0.1 oz increments.
(iv) If a manufacturer elects to use abbreviations for units, the following abbreviations shall be used: tbsp for tablespoon, tsp for teaspoon, g for gram, mL for milliliter, oz for ounce, and fl oz for fluid ounce.
(v) For products that only require the addition of water or another ingredient that contains insignificant amounts of nutrients in the amount added and that are prepared in such a way that there are no significant changes to the nutrient profile, the amount of the finished product may be declared in parentheses at the end of the serving size declaration (e.g.,
1/2 cup (120 mL) concentrated soup (makes 1 cup prepared)).
(vi) To promote uniformity in label serving sizes in household measures declared by different manufacturers, FDA has provided a guidance document entitled, "Guidelines for Determining the Gram Weight of the Household Measure." The guidance document can be obtained from the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740.
(8) Determination of the number of servings per container shall be based on the serving size of the product determined by following the procedures described in this section.
(i) The number of servings shall be rounded to the nearest whole number except for the number of servings between 2 and 5 servings and random weight products. The number of servings between 2 and 5 servings shall be rounded to the nearest 0.5 serving. Rounding should be indicated by the use of the term about (e.g., about 2 servings, about 3.5 servings).
(ii) When the serving size is required to be expressed on a drained solids basis and the number of servings varies because of a natural variation in unit size (e.g., maraschino cherries, pickles), the manufacturer may state the typical number of servings per container (e.g., usually 5 servings).
(iii) For random weight products, manufacturers may declare "varied" for the number of servings per container provided the nutrition information is based on the reference amount expressed in the appropriate household measure based on the hierarchy described in paragraph (b)(5) of this section. Random weight products are foods such as cheeses that are sold as random weights that vary in size, such that the net contents for different containers would vary. The manufacturer may provide the typical number of servings in parentheses following the "varied" statement.
(iv) For packages containing several individual single-serving containers, each of which is labeled with all required information including nutrition labeling as specified in § 101.9 (that is, are labeled appropriately for individual sale as single-serving containers), the number of servings shall be the number of individual packages within the total package.
(v) For packages containing several individually packaged multiserving units, the number of servings shall be determined by multiplying the number of individual multiserving units in the total package by the number of servings in each individual unit.
(9) The declaration of nutrient and food component content shall be on the basis of food as packaged or purchased with the exception of raw fish covered under § 101.42 (see 101.44), packaged single-ingredient products that consist of fish or game meat as provided for in paragraph (j)(11) of this section, and of foods that are packed or canned in water, brine, or oil but whose liquid packing medium is not customarily consumed (e.g., canned fish, maraschino cherries, pickled fruits, and pickled vegetables). Declaration of nutrient and food component content of raw fish shall follow the provisions in § 101.45. Declaration of the nutrient and food component content of foods that are packed in liquid which is not customarily consumed shall be based on the drained solids.
(10) Another column of figures may be used to declare the nutrient and food component information:
(i) Per 100 g or 100 mL, or per 1 oz or 1 fl oz of the food as packaged or purchased;
(ii) Per one unit if the serving size of a product in discrete units is more than 1 unit.
(iii) Per cup popped for popcorn in a multiserving container.
(11) If a product is promoted on the label, labeling, or advertising for a use that differs in quantity by twofold or greater from the use upon which the reference amount in § 101.12(b) was based (e.g., liquid cream substitutes promoted for use with breakfast cereals), the manufacturer shall provide a second column of nutrition information based on the amount customarily consumed in the promoted use, in addition to the nutrition information per serving derived from the reference amount in § 101.12(b), except that nondiscrete bulk products that are used primarily as ingredients (e.g., flour, sweeteners, shortenings, oils), or traditionally used for multipurposes (e.g., eggs, butter, margarine), and multipurpose baking mixes are exempt from this requirement.
(12)(i) Products that are packaged and sold individually and that contain at least 200 percent and up to and including 300 percent of the applicable reference amount must provide an additional column within the Nutrition Facts label that lists the quantitative amounts and percent Daily Values for the entire package, as well as a column listing the quantitative amounts and percent Daily Values for a serving that is less than the entire package (i.e., the serving size derived from the reference amount). The first column would be based on the serving size for the product and the second column would be based on the entire contents of the package.
(A) This provision does not apply to products that meet the requirements to use the tabular format in paragraph (j)(13)(ii)(A)(1 ) of this section or to products that meet the requirements to use the linear format in paragraph (j)(13)(ii)(A)(2 ) of this section.
(B) This provision does not apply to raw fruits, vegetables, and seafood for which voluntary nutrition labeling is provided in the product labeling or advertising or when claims are made about the product.
(C) This provision does not apply to products that require further preparation and provide an additional column of nutrition information under paragraph (e) of this section, to products that are commonly consumed in combination with another food and provide an additional column of nutrition information under paragraph (e) of this section, to products that provide an additional column of nutrition information for two or more groups for which RDIs are established (e.g., both infants and children less than 4 years of age), to popcorn products that provide an additional column of nutrition information per 1 cup popped popcorn, or to varied-weight products covered under paragraph (b)(8)(iii) of this section.
(ii) When a nutrient content claim or health claim is made on the label of a product that uses a dual column as required in paragraph (b)(2)(i)(D) or (b)(12)(i) of this section, the claim must be followed by a statement that sets forth the basis on which the claim is made, except that the statement is not required for products when the nutrient that is the subject of the claim meets the criteria for the claim based on the reference amount for the product and the entire container or the unit amount. When a nutrient content claim is made, the statement must express that the claim refers to the amount of the nutrient per serving (e.g., "good source of calcium per serving" or "per X [insert unit]__serving") or per reference amount (e.g., "good source of calcium per [insert reference amount (e.g., per 8 ounces)]), as required based on § 101.12(g). When a health claim is made, the statement shall be "A serving of __ounces of this product conforms to such a diet."
(c) The declaration of nutrition information on the label and in labeling of a food shall contain information about the level of the following nutrients, except for those nutrients whose inclusion, and the declaration of amounts, is voluntary as set forth in this paragraph. No nutrients or food components other than those listed in this paragraph as either mandatory or voluntary may be included within the nutrition label. Except as provided for in paragraphs (f) or (j) of this section, nutrient information shall be presented using the nutrient names specified and in the following order in the formats specified in paragraphs (d) or (e) of this section.
(1) "Calories, total," "Total calories," or "Calories": A statement of the caloric content per serving, expressed to the nearest 5-calorie increment up to and including 50 calories, and 10-calorie increment above 50 calories, except that amounts less than 5 calories may be expressed as zero. Energy content per serving may also be expressed in kilojoule units, added in parentheses immediately following the statement of the caloric content.
(i) Caloric content may be calculated by the following methods. Where either specific or general food factors are used, the factors shall be applied to the actual amount (i.e., before rounding) of food components (e.g., fat, carbohydrate, protein, or ingredients with specific food factors) present per serving.
(A) Using specific Atwater factors (i.e., the Atwater method) given in table 13, USDA Handbook No. 74 (slightly revised, 1973),
(B) Using the general factors of 4, 4, and 9 calories per gram for protein, total carbohydrate, and total fat, respectively, as described in USDA Handbook No. 74 (slightly revised, 1973) pp. 9-11;
(C) Using the general factors of 4, 4, and 9 calories per gram for protein, total carbohydrate (less the amount of non-digestible carbohydrates and sugar alcohols), and total fat, respectively, as described in USDA Handbook No. 74 (slightly revised, 1973) pp. 9-11. A general factor of 2 calories per gram for soluble non-digestible carbohydrates shall be used. The general factors for caloric value of sugar alcohols provided in paragraph (c)(1)(i)(F) of this section shall be used;
(D) Using data for specific food factors for particular foods or ingredients approved by the Food and Drug Administration (FDA) and provided in parts 172 or 184 of this chapter, or by other means, as appropriate;
(E) Using bomb calorimetry data subtracting 1.25 calories per gram protein to correct for incomplete digestibility, as described in USDA Handbook No. 74 (slightly revised, 1973) p. 10; or
(F) Using the following general factors for caloric value of sugar alcohols: Isomalt - 2.0 calories per gram, lactitol - 2.0 calories per gram, xylitol - 2.4 calories per gram, maltitol - 2.1 calories per gram, sorbitol - 2.6 calories per gram, hydrogenated starch hydrolysates - 3.0 calories per gram, mannitol - 1.6 calories per gram, and erythritol - 0 calories per gram.
(ii) "Calories from saturated fat" or "Calories from saturated" (VOLUNTARY): A statement of the caloric content derived from saturated fat as defined in paragraph (c)(2)(i) of this section in a serving may be declared voluntarily, expressed to the nearest 5-calorie increment, up to and including 50 calories, and the nearest 10-calorie increment above 50 calories, except that amounts less than 5 calories may be expressed as zero. This statement shall be indented under the statement of calories as provided in paragraph (d)(5) of this section.
(2) "Fat, total" or "Total fat": A statement of the number of grams of total fat in a serving defined as total lipid fatty acids and expressed as triglycerides where fatty acids are aliphatic carboxylic acids consisting of a chain of alkyl groups and characterized by a terminal carboxyl group. Amounts shall be expressed to the nearest 0.5 (
1/2) gram increment below 5 grams and to the nearest gram increment above 5 grams. If the serving contains less than 0.5 gram, the content shall be expressed as zero.
(i) "Saturated fat," or "Saturated": A statement of the number of grams of saturated fat in a serving defined as the sum of all fatty acids containing no double bonds, except that label declaration of saturated fat content information is not required for products that contain less than 0.5 gram of total fat in a serving if no claims are made about fat, fatty acid, or cholesterol content, and if "calories from saturated fat" is not declared. Except as provided for in paragraph (f) of this section, if a statement of the saturated fat content is not required and, as a result, not declared, the statement "Not a significant source of saturated fat" shall be placed at the bottom of the table of nutrient values. Saturated fat content shall be indented and expressed as grams per serving to the nearest 0.5 gram (
1/2) gram increment below 5 grams and to the nearest gram increment above 5 grams. If the serving contains less than 0.5 gram, the content shall be expressed as zero.
(ii) "Trans fat" or "Trans": A statement of the number of grams of trans fat in a serving, defined as the sum of all unsaturated fatty acids that contain one or more isolated (i.e., nonconjugated) double bonds in a trans configuration, except that label declaration of trans fat content information is not required for products that contain less than 0.5 gram of total fat in a serving if no claims are made about fat, fatty acid or cholesterol content. The word "trans" may be italicized to indicate its Latin origin. Trans fat content shall be indented and expressed as grams per serving to the nearest 0.5 (
1/2)-gram increment below 5 grams and to the nearest gram increment above 5 grams. If the serving contains less than 0.5 gram, the content, when declared, shall be expressed as zero. Except as provided for in paragraph (f) of this section, if a statement of the trans fat content is not required and, as a result, not declared, the statement "Not a significant source of trans fat" shall be placed at the bottom of the table of nutrient values.
(iii) "Polyunsaturated fat" or "Poly-unsaturated" (VOLUNTARY): A statement of the number of grams of polyunsaturated fat in a serving defined as cis,cis-methylene-interrupted polyunsaturated fatty acids may be declared voluntarily, except that when monounsaturated fat is declared, or when a claim about fatty acids or cholesterol is made on the label or in labeling of a food other than one that meets the criteria in § 101.62(b)(1) for a claim for "fat free," label declaration of polyunsaturated fat is required. Polyunsaturated fat content shall be indented and expressed as grams per serving to the nearest 0.5 (
1/2) gram increment below 5 grams and to the nearest gram increment above 5 grams. If the serving contains less than 0.5 gram, the content shall be expressed as zero.
(iv) "Monounsaturated fat" or "Monounsaturated" (VOLUNTARY): A statement of the number of grams of monounsaturated fat in a serving defined as cis-monounsaturated fatty acids may be declared voluntarily except that when polyunsaturated fat is declared, or when a claim about fatty acids or cholesterol is made on the label or in labeling of a food other than one that meets the criteria in § 101.62(b)(1) for a claim for "fat free," label declaration of monounsaturated fat is required. Monounsaturated fat content shall be indented and expressed as grams per serving to the nearest 0.5 (
1/2) gram increment below 5 grams and to the nearest gram increment above 5 grams. If the serving contains less than 0.5 gram, the content shall be expressed as zero.
(3) "Cholesterol": A statement of the cholesterol content in a serving expressed in milligrams to the nearest 5-milligram increment, except that label declaration of cholesterol information is not required for products that contain less than 2 milligrams cholesterol in a serving and make no claim about fat, fatty acids, or cholesterol content, or such products may state the cholesterol content as zero. Except as provided for in paragraph (f) of this section, if cholesterol content is not required and, as a result, not declared, the statement "Not a significant source of cholesterol" shall be placed at the bottom of the table of nutrient values in the same type size. If the food contains 2 to 5 milligrams of cholesterol per serving, the content may be stated as "less than 5 milligrams."
(4) "Sodium": A statement of the number of milligrams of sodium in a specified serving of food expressed as zero when the serving contains less than 5 milligrams of sodium, to the nearest 5-milligram increment when the serving contains 5 to 140 milligrams of sodium, and to the nearest 10-milligram increment when the serving contains greater than 140 milligrams.
(5) "Fluoride" (VOLUNTARY): A statement of the number of milligrams of fluoride in a specified serving of food may be declared voluntarily, except that when a claim is made about fluoride content, label declaration shall be required. Fluoride content shall be expressed as zero when the serving contains less than 0.1 milligrams of fluoride, to the nearest 0.1-milligram increment when the serving contains less than or equal to 0.8 milligrams of fluoride, and the nearest 0.2 milligram-increment when a serving contains more than 0.8 milligrams of fluoride. Bottled water that bears a statement about added fluoride, as permitted by § 101.13(q)(8), must bear nutrition labeling that complies with requirements for the simplified format in paragraph (f) of this section.
(6) "Carbohydrate, total" or "Total carbohydrate": A statement of the number of grams of total carbohydrate in a serving expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, or if the serving contains less than 0.5 gram, the content may be expressed as zero. Total carbohydrate content shall be calculated by subtraction of the sum of the crude protein, total fat, moisture, and ash from the total weight of the food. This calculation method is described in A. L. Merrill and B. K. Watt, "Energy Value of Foods - Basis and Derivation," USDA Handbook 74 (slightly revised 1973) pp. 2 and 3, which is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51 (the availability of this incorporation by reference is given in paragraph (c)(1)(i)(A) of this section).
(i) "Dietary fiber": A statement of the number of grams of total dietary fiber in a serving, indented and expressed to the nearest gram, except that if a serving contains less than 1 gram, declaration of dietary fiber is not required or, alternatively, the statement "Contains less than 1 gram" or "less than 1 gram" may be used, and if the serving contains less than 0.5 gram, the content may be expressed as zero. Dietary fiber is defined as non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health. Except as provided for in paragraph (f) of this section, if dietary fiber content is not required, and as a result not declared, the statement "Not a significant source of dietary fiber" shall be placed at the bottom of the table of nutrient values in the same type size. The following isolated or synthetic nondigestible carbohydrate(s) have been determined by FDA to have physiological effects that are beneficial to human health and, therefore, shall be included in the calculation of the amount of dietary fiber: [beta]-glucan soluble fiber (as described in § 101.81(c)(2)(ii)(A)), psyllium husk (as described in § 101.81(c)(2)(ii)(B)(1 )), cellulose, guar gum, pectin, locust bean gum, and hydroxypropylmethylcellulose. The manufacturer must make and keep records in accordance with paragraphs (g)(10) and (11) of this section to verify the declared amount of dietary fiber in the label and labeling of food when a mixture of dietary fiber, and added nondigestible carbohydrate(s) that does not meet the definition of dietary fiber, is present in the food.
(A) "Soluble fiber" (VOLUNTARY): A statement of the number of grams of soluble dietary fiber in a serving may be declared voluntarily except that when a claim is made on the label or in labeling about soluble fiber, label declaration shall be required. Soluble fiber must meet the definition of dietary fiber in this paragraph (c)(6)(i). The manufacturer must make and keep records in accordance with paragraphs (g)(10) and (11) of this section to verify the declared amount of soluble fiber in the label and labeling of food when a mixture of soluble fiber and added non-digestible carbohydrate(s) that does not meet the definition of dietary fiber is present in the food. Soluble fiber content shall be indented under dietary fiber and expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero."
(B) "Insoluble fiber" (VOLUNTARY): A statement of the number of grams of insoluble dietary fiber in a serving may be declared voluntarily except that when a claim is made on the label or in labeling about insoluble fiber, label declaration shall be required. Insoluble fiber must meet the definition of dietary fiber in this paragraph (c)(6)(i). The manufacturer must make and keep records in accordance with paragraphs (g)(10) and (11) of this section to verify the declared amount of insoluble fiber in the label and labeling of food when a mixture of insoluble and added non-digestible carbohydrate(s) that does not meet the definition of dietary fiber is present in the food. Insoluble fiber content shall be indented under dietary fiber and expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero.
(ii) "Total Sugars": A statement of the number of grams of sugars in a serving, except that the label declaration of sugars content is not required for products that contain less than 1 gram of sugars in a serving if no claims are made about sweeteners, sugars, or sugar alcohol content. Except as provided for in paragraph (f) of this section, if a statement of the total sugars content is not required and, as a result, not declared, the statement "Not a significant source of total sugars" shall be placed at the bottom of the table of nutrient values in the same type size. Total sugars shall be defined as the sum of all free mono- and disaccharides (such as glucose, fructose, lactose, and sucrose). Total sugars content shall be indented and expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero.
(iii) "Added Sugars": A statement of the number of grams of added sugars in a serving, except that label declaration of added sugars content is not required for products that contain less than 1 gram of added sugars in a serving if no claims are made about sweeteners, sugars, added sugars, or sugar alcohol content. Except as provided for in paragraph (f) of this section, if a statement of the added sugars content is not required and, as a result, not declared, the statement "Not a significant source of added sugars" shall be placed at the bottom of the table of nutrient values in the same type size. Added sugars are either added during the processing of foods, or are packaged as such, and include sugars (free, mono and disaccharides), sugars from syrups and honey, and sugars from concentrated fruit or vegetable juices that are in excess of what would be expected from the same volume of 100 percent fruit or vegetable juice of the same type, except that fruit or vegetable juice concentrated from 100 percent juices sold to consumers, fruit or vegetable juice concentrates used towards the total juice percentage label declaration under § 101.30 or for Brix standardization under § 102.33(g)(2) of this chapter, fruit juice concentrates which are used to formulate the fruit component of jellies, jams, or preserves in accordance with the standard of identities set forth in §§ 150.140 and 150.160 of this chapter, or the fruit component of fruit spreads shall not be labeled as added sugars. Added sugars content shall be indented under Total Sugars and shall be prefaced with the word "Includes" followed by the amount (in grams) "Added Sugars" ("Includes `X' g Added Sugars"). It shall be expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero. When a mixture of naturally occurring and added sugars is present in the food, and for specific foods containing added sugars, alone or in combination with naturally occurring sugars, where the added sugars are subject to fermentation and/or non-enzymatic browning, the manufacturer must make and keep records in accordance with paragraphs (g)(10) and (11) of this section to verify the declared amount of added sugars in the label and labeling of food.
(iv) "Sugar alcohol" (VOLUNTARY): A statement of the number of grams of sugar alcohols in a serving may be declared voluntarily on the label, except that when a claim is made on the label or in labeling about sugar alcohol or total sugars, or added sugars when sugar alcohols are present in the food, sugar alcohol content shall be declared. For nutrition labeling purposes, sugar alcohols are defined as the sum of saccharide derivatives in which a hydroxyl group replaces a ketone or aldehyde group and whose use in the food is listed by FDA (e.g., mannitol or xylitol) or is generally recognized as safe (e.g., sorbitol). In lieu of the term "sugar alcohol," the name of the specific sugar alcohol (e.g., "xylitol") present in the food may be used in the nutrition label provided that only one sugar alcohol is present in the food. Sugar alcohol content shall be indented and expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero.
(7) "Protein": A statement of the number of grams of protein in a serving, expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement "Contains less than 1 gram" or "less than 1 gram" may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero. When the protein in foods represented or purported to be for adults and children 4 or more years of age has a protein quality value that is a protein digestibility-corrected amino acid score of less than 20 expressed as a percent, or when the protein in a food represented or purported to be for children greater than 1 but less than 4 years of age has a protein quality value that is a protein digestibility-corrected amino acid score of less than 40 expressed as a percent, either of the following shall be placed adjacent to the declaration of protein content by weight: The statement "not a significant source of protein," or a listing aligned under the column headed "Percent Daily Value" of the corrected amount of protein per serving, as determined in paragraph (c)(7)(ii) of this section, calculated as a percentage of the Daily Reference Value (DRV) or Reference Daily Intake (RDI), as appropriate, for protein and expressed as a Percent of Daily Value. When the protein quality in a food as measured by the Protein Efficiency Ratio (PER) is less than 40 percent of the reference standard (casein) for a food represented or purported to be specifically for infants through 12 months, the statement "not a significant source of protein" shall be placed adjacent to the declaration of protein content. Protein content may be calculated on the basis of the factor 6.25 times the nitrogen content of the food as determined by the appropriate method of analysis as given in the "Official Methods of Analysis of the AOAC International," except when official AOAC procedures described in this paragraph (c)(7) require a specific factor other than 6.25, that specific factor shall be used.
(i) A statement of the corrected amount of protein per serving, as determined in paragraph (c)(7)(ii) of this section, calculated as a percentage of the RDI or DRV for protein, as appropriate, and expressed as Percent of Daily Value, may be placed on the label, except that such a statement shall be given if a protein claim is made for the product, or if the product is represented or purported to be specifically for infants through 12 months or children 1 through 3 years of age. When such a declaration is provided, it should be placed on the label adjacent to the statement of grams of protein and aligned under the column headed "Percent Daily Value," and expressed to the nearest whole percent. However, the percentage of the RDI for protein shall not be declared if the food is represented or purported to be specifically for infants through 12 months and the protein quality value is less than 40 percent of the reference standard.
(ii) The "corrected amount of protein (gram) per serving" for foods represented or purported for adults and children 1 or more years of age is equal to the actual amount of protein (gram) per serving multiplied by the amino acid score corrected for protein digestibility. If the corrected score is above 1.00, then it shall be set at 1.00. The protein digestibility-corrected amino acid score shall be determined by methods given in sections 5.4.1, 7.2.1, and 8.00 in "Report of the Joint FAO/WHO Expert Consultation on Protein Quality Evaluation," except that when official AOAC procedures described in paragraph (c)(7) of this section require a specific factor other than 6.25, that specific factor shall be used. For foods represented or purported to be specifically for infants through 12 months, the corrected amount of protein (grams) per serving is equal to the actual amount of protein (grams) per serving multiplied by the relative protein quality value. The relative protein quality value shall be determined by dividing the subject food protein PER value by the PER value for casein. If the relative protein value is above 1.00, it shall be set at 1.00.
(iii) For the purpose of labeling with a percent of the DRV or RDI, a value of 50 grams of protein shall be the DRV for adults and children 4 or more years of age, a value of 11 grams of protein shall be the RDI for infants through 12 months, a value of 13 grams shall be the DRV for children 1 through 3 years of age, and a value of 71 grams of protein shall be the RDI for pregnant women and lactating women.
(8) "Vitamins and minerals": The requirements related to including a statement of the amount per serving of vitamins and minerals are described in this paragraph (c)(8).
(i) For purposes of declaration of percent of Daily Value as provided for in paragraphs (d), (e), and (f) of this section, foods represented or purported to be specifically for infants through 12 months, children 1 through 3 years, pregnant women, and lactating women shall use the RDIs that are specified for the intended group. For foods represented or purported to be specifically for both infants through 12 months of age and children 1 through 3 years of age, the percent of Daily Value shall be presented by separate declarations according to paragraph (e) of this section based on the RDI values for infants through 12 months of age and children 1 through 3 years of age. When such dual declaration is used on any label, it shall be included in all labeling, and equal prominence shall be given to both values in all such labeling. The percent Daily Value based on the RDI values for pregnant women and lactating women shall be declared on food represented or purported to be specifically for pregnant women and lactating women. All other foods shall use the RDI for adults and children 4 or more years of age.
(ii) The declaration of vitamins and minerals as a quantitative amount by weight and percent of the RDI shall include vitamin D, calcium, iron, and potassium in that order, for infants through 12 months, children 1 through 3 years of age, pregnant women, lactating women, and adults and children 4 or more years of age, except quantitative weights for these vitamins and minerals are not required for labels described in paragraph (j)(13) of this section. The declaration of folic acid shall be included as a quantitative amount by weight when added as a nutrient supplement or a claim is made about the nutrient. The declaration of vitamins and minerals in a food, as a quantitative amount by weight and percent of the RDI, may include any of the other vitamins and minerals listed in paragraph (c)(8)(iv) of this section. The declaration of vitamins and minerals shall include any of the other vitamins and minerals listed in paragraph (c)(8)(iv) of this section as a statement of the amount per serving of the vitamins and minerals as described in this paragraph (c)(8)(ii), calculated as a percent of the RDI and expressed as a percent of the Daily Value, when they are added as a nutrient supplement, or when a claim is made about them, unless otherwise stated as quantitative amount by weight and percent of the Daily Value. Other vitamins and minerals need not be declared if neither the nutrient nor the component is otherwise referred to on the label or the labeling or advertising and the vitamins and minerals are:
(A) Required or permitted in a standardized food (e.g., thiamin, riboflavin, and niacin in enriched flour) and that standardized food is included as an ingredient (i.e., component) in another food; or
(B) Included in a food solely for technological purposes and declared only in the ingredient statement. The declaration may also include any of the other vitamins and minerals listed in paragraph (c)(8)(iv) of this section when they are naturally occurring in the food. The additional vitamins and minerals shall be listed in the order established in paragraph (c)(8)(iv) of this section.
(iii) The percentages for vitamins and minerals shall be expressed to the nearest 2-percent increment up to and including the 10-percent level, the nearest 5-percent increment above 10 percent and up to and including the 50-percent level, and the nearest 10-percent increment above the 50-percent level. Quantitative amounts and percentages of vitamins and minerals present at less than 2 percent of the RDI are not required to be declared in nutrition labeling but may be declared by a zero or by the use of an asterisk (or other symbol) that refers to another asterisk (or symbol) that is placed at the bottom of the table and that is followed by the statement "Contains less than 2 percent of the Daily Value of this (these) nutrient (nutrients)" or "Contains <2 percent of the Daily Value of this (these) nutrient (nutrients)." Alternatively, except as provided for in paragraph (f) of this section, if vitamin D, calcium, iron, or potassium is present in amounts less than 2 percent of the RDI, label declaration of the nutrient(s) is not required if the statement "Not a significant source of - (listing the vitamins or minerals omitted)" is placed at the bottom of the table of nutrient values. Either statement shall be in the same type size as nutrients that are indented. The quantitative amounts of vitamins and minerals, excluding sodium, shall be the amount of the vitamin or mineral included in one serving of the product, using the units of measurement and the levels of significance given in paragraph (c)(8)(iv) of this section, except that zeros following decimal points may be dropped, and additional levels of significance may be used when the number of decimal places indicated is not sufficient to express lower amounts (e.g., the RDI for zinc is given in whole milligrams, but the quantitative amount may be declared in tenths of a milligram).
(iv) The following RDIs, nomenclature, and units of measure are established for the following vitamins and minerals which are essential in human nutrition:
|
| RDI
|
|---|
| Nutrient
| Unit of measure
| Adults and children greater than or equal to 4 years
| Infants
1 through 12 months
| Children 1 through 3 years
| Pregnant women and lactating women
|
|---|
| Vitamin D | Micrograms (mcg)
2 | 20 | 10 | 15 | 15
| | Calcium | Milligrams (mg) | 1,300 | 260 | 700 | 1,300
| | Iron | Milligrams (mg) | 18 | 11 | 7 | 27
| | Potassium | Milligrams (mg) | 4,700 | 700 | 3,000 | 5,100
| | Vitamin A | Micrograms RAE
3 (mcg) | 900 | 500 | 300 | 1,300
| | Vitamin C | Milligrams (mg) | 90 | 50 | 15 | 120
| | Vitamin E | Milligrams (mg)
4 | 15 | 5 | 6 | 19
| | Vitamin K | Micrograms (mcg) | 120 | 2.5 | 30 | 90
| | Thiamin | Milligrams (mg) | 1.2 | 0.3 | 0.5 | 1.4
| | Riboflavin | Milligrams (mg) | 1.3 | 0.4 | 0.5 | 1.6
| | Niacin | Milligrams NE
5 (mg) | 16 | 4 | 6 | 18
| | Vitamin B6 | Milligrams (mg) | 1.7 | 0.3 | 0.5 | 2.0
| | Folate
6 | Micrograms DFE
7 (mcg) | 400 | 80 | 150 | 600
| | Vitamin B12 | Micrograms (mcg) | 2.4 | 0.5 | 0.9 | 2.8
| | Biotin | Micrograms (mcg) | 30 | 6 | 8 | 35
| | Pantothenic acid | Milligrams (mg) | 5 | 1.8 | 2 | 7
| | Phosphorus | Milligrams (mg) | 1,250 | 275 | 460 | 1,250
| | Iodine | Micrograms (mcg) | 150 | 130 | 90 | 290
| | Magnesium | Milligrams (mg) | 420 | 75 | 80 | 400
| | Zinc | Milligrams (mg) | 11 | 3 | 3 | 13
| | Selenium | Micrograms (mcg) | 55 | 20 | 20 | 70
| | Copper | Milligrams (mg) | 0.9 | 0.2 | 0.3 | 1.3
| | Manganese | Milligrams (mg) | 2.3 | 0.6 | 1.2 | 2.6
| | Chromium | Micrograms (mcg) | 35 | 5.5 | 11 | 45
| | Molybdenum | Micrograms (mcg) | 45 | 3 | 17 | 50
| | Chloride | Milligrams (mg) | 2,300 | 570 | 1,500 | 2,300
| | Choline | Milligrams (mg) | 550 | 150 | 200 | 550
| | Protein | Grams (g) | N/A | 11 | N/A |
8 71
|
(v) The following synonyms may be added in parentheses immediately following the name of the nutrient or dietary component:
Calories - Energy
Vitamin C - Ascorbic acid
Thiamin - Vitamin B1
Riboflavin - Vitamin B2
(vi) A statement of the percent of vitamin A that is present as beta -carotene may be declared voluntarily. When the vitamins and minerals are listed in a single column, the statement shall be indented under the information on vitamin A. When vitamins and minerals are arrayed horizontally, the statement of percent shall be presented in parenthesis following the declaration of vitamin A and the percent DV of vitamin A in the food (e.g., "Percent Daily Value: Vitamin A 50 (90 percent as beta -carotene)"). When declared, the percentages shall be expressed in the same increments as are provided for vitamins and minerals in paragraph (c)(8)(iii) of this section.
(vii) When the amount of folate is declared in the labeling of a conventional food or a dietary supplement, the nutrient name "folate" shall be listed for products containing folate (natural folate, and/or synthetic folate as a component of dietary supplement, such as calcium salt of L-5-MTHF), folic acid, or a mixture of folate and folic acid. The name of the synthetic form of the nutrient "folic acid", when added or a claim is made about the nutrient, shall be included in parentheses after this declaration with the amount of folic acid. The declaration must be folate in mcg DFE (when expressed as a quantitative amount by weight in a conventional food or a dietary supplement) and the percent DV based on folate in mcg DFE, or for conventional food, may be expressed as folate and the percent DV based on folate in mcg DFE. When declared, folic acid must be in parentheses, mcg of folic acid as shown in paragraph (d)(12) of this section in the display that illustrates voluntary declaration of nutrition information.
(9) The following DRVs, nomenclature, and units of measure are established for the following food components:
| Food component
| Unit of measure
| Adults and children greater than or equal to 4 years
| Infants through 12 months
| Children 1 through 3 years
| Pregnant women and lactating women
|
|---|
| Fat | Grams (g) |
1 78 | 30 |
2 39 |
1 78
| | Saturated fat | Grams (g) |
1 20 | N/A |
2 10 |
1 20
| | Cholesterol | Milligrams (mg) | 300 | N/A | 300 | 300
| | Total carbohydrate | Grams (g) |
1 275 | 95 |
2 150 |
1 275
| | Sodium | Milligrams (mg) | 2,300 | N/A | 1,500 | 2,300
| | Dietary Fiber | Grams (g) |
1 28 | N/A |
2 14 |
1 28
| | Protein | Grams (g) |
1 50 | N/A |
2 13 | N/A
| | Added Sugars | Grams (g) |
1 50 | N/A |
2 25 |
1 50
|
(d)(1) Nutrient information specified in paragraph (c) of this section shall be presented on foods in the following format, as shown in paragraph (d)(12) of this section, except on foods where the tabular display is permitted as provided for in paragraph (d)(11) of this section, on which dual columns of nutrition information are declared as provided for in paragraph (e) of this section, on those food products on which the simplified format is required to be used as provided for in paragraph (f) of this section, on foods for infants through 12 months of age and children 1 through 3 years of age as provided for in paragraph (j)(5) of this section, and on foods in small or intermediate-sized packages as provided for in paragraph (j)(13) of this section. In the interest of uniformity of presentation, FDA strongly recommends that the nutrition information be presented using the graphic specifications set forth in appendix B to part 101.
(i) The nutrition information shall be set off in a box by use of hairlines and shall be all black or one color type, printed on a white or other neutral contrasting background whenever practical.
(ii) All information within the nutrition label shall utilize:
(A) Except as provided for in paragraph (c)(2)(ii) of this section, a single easy-to-read type style,
(B) Upper and lower case letters,
(C) At least one point leading (i.e., space between two lines of text) except that at least four points leading shall be utilized for the information required by paragraphs (d)(7) and (d)(8) of this section as shown in paragraph (d)(12), and
(D) Letters should never touch.
(iii) Information required in paragraphs (d)(7) and (8) of this section shall be in type size no smaller than 8 point. Information required in paragraph (d)(5) of this section for the "Calories" declaration shall be highlighted in bold or extra bold and shall be in a type size no smaller than 16 point except the type size for this information required in the tabular displays as shown in paragraphs (d)(11), (e)(6)(ii), and (j)(13)(ii)(A)(1 ) of this section and the linear display for small packages as shown in paragraph (j)(13)(ii)(A)(2 ) of this section shall be in a type size no smaller than 10 point. The numeric amount for the information required in paragraph (d)(5) of this section shall also be highlighted in bold or extra bold type and shall be in a type size no smaller than 22 point, except the type size for this information required for the tabular display for small packages as shown in paragraph (j)(13)(ii)(A)(1 ) of this section, and for the linear display for small packages as shown in paragraph (j)(13)(ii)(A)(2 ) of this section no smaller than 14 point. The information required in paragraphs (d)(4), (6), and (9) of this section shall be in a type size no smaller than 6 point. When provided, the information described in paragraph (d)(10) of this section shall be in a type size no smaller than 6 point.
(iv) The headings required by paragraphs (d)(2), (d)(3)(ii), (d)(4), and (d)(6) of this section (i.e., "Nutrition Facts," "Serving size," "Amount per serving," and "% Daily Value*"), the names of all nutrients that are not indented according to requirements of paragraph (c) of this section (i.e., "Calories," "Total Fat," "Cholesterol," "Sodium," "Total Carbohydrate" and "Protein"), and the percentage amounts required by paragraph (d)(7)(ii) of this section shall be highlighted in bold or extra bold type or other highlighting (reverse printing is not permitted as a form of highlighting) that prominently distinguishes it from other information. No other information shall be highlighted.
(v) A hairline rule that is centered between the lines of text shall separate "Nutrition Facts" from the servings per container statement required in paragraph (d)(3)(i) of this section and shall separate each nutrient and its corresponding percent Daily Value required in paragraphs (d)(7)(i) and (ii) of this section from the nutrient and percent Daily Value above and below it, as shown in paragraph (d)(12) of this section and in Appendix B to Part 101.
(2) The information shall be presented under the identifying heading of "Nutrition Facts" which shall be set in a type size no smaller than all other print size in the nutrition label except for the numerical information for "Calories" required in paragraph (d)(5) of this section, and except for labels presented according to the format provided for in paragraphs (d)(11), (d)(13)(ii), (e)(6)(ii), (j)(13)(ii)(A)(1 ), and (j)(13)(ii)(A)(2 ) of this section, unless impractical, shall be set the full width of the information provided under paragraph (d)(7) of this section, as shown in paragraph (d)(12) of this section.
(3) Information on servings per container and serving size shall immediately follow the heading as shown in paragraph (d)(12) of this section. Such information shall include:
(i) "____ servings per container": The number of servings per container, except that this statement is not required on single serving containers as defined in paragraph (b)(6) of this section or on other food containers when this information is stated in the net quantity of contents declaration. The information required in this paragraph shall be located immediately after the "Nutrition Facts" heading and shall be in a type size no smaller than 10 point, except the type size for this information shall be no smaller than 9 point in the tabular display for small packages as shown in paragraph (j)(13)(ii)(A)(1 ) of this section and the linear display for small packages as shown in paragraph (j)(13)(ii)(A)(2 ) of this section. For the linear display for small packages as shown in paragraph (j)(13)(ii)(A)(2 ) of this section, the actual number of servings may be listed after the servings per container declaration.
(ii) "Serving size": A statement of the serving size as specified in paragraph (b)(7) of this section which shall immediately follow the "____servings per container" declaration. The information required in this paragraph shall be highlighted in bold or extra bold and be in a type size no smaller than 10 point, except the type size shall be no smaller than 9 point for this information in the tabular displays as shown in paragraphs (d)(11) and (e)(6)(ii) of this section, the tabular display for small packages as shown in paragraph (j)(13)(ii)(A)(1 ) of this section, and the linear display for small packages as shown in paragraph (j)(13)(ii)(A)(2 ) of this section. The serving size amount must be right justified if adequate space is available. If the "Serving size" declaration does not fit in the allocated space a type size of no smaller than 8 point may be used on packages of any size.
(4) A subheading "Amount per serving" shall be separated from the serving size information by a bar as shown in paragraph (d)(12) of this section, except this information is not required for the dual column formats shown in paragraphs (e)(5), (e)(6)(i), and (e)(6)(ii) of this section.
(5) Information on calories shall immediately follow the subheading "Amount per serving" and shall be declared in one line. If "Calories from saturated fat" is declared, it shall be indented under "Calories" and shall be in a type size no smaller than 8 point.
(6) The column heading "% Daily Value," followed by an asterisk (e.g., "% Daily Value*"), shall be separated from information on calories by a bar as shown in paragraph (d)(12) of this section. The position of this column heading shall allow for a list of nutrient names and amounts as described in paragraph (d)(7) of this section to be to the left of, and below, this column heading. The column headings "Percent Daily Value," "Percent DV," or "% DV" may be substituted for "% Daily Value."
(7) Except as provided for in paragraph (j)(13)(ii)(A)(2 ) of this section, nutrient information for both mandatory and any voluntary nutrients listed in paragraph (c) of this section that are to be declared in the nutrition label, except for folic acid in conventional food and voluntarily declared vitamins and minerals expressed as a statement of the amount per serving calculated as a percent of the RDI and expressed as a percent Daily Value, shall be declared as follows:
(i) The name of each nutrient, as specified in paragraph (c) of this section, shall be given in a column and followed immediately by the quantitative amount by weight for that nutrient appended with a "g" for grams, "mg" for milligrams, or "mcg" for micrograms as shown in paragraph (d)(12) of this section. The symbol "<" may be used in place of "less than."
(ii) A listing of the percent of the DRV as established in paragraphs (c)(7)(iii) and (c)(9) of this section shall be given in a column aligned under the heading "% Daily Value" established in paragraph (d)(6) of this section with the percent expressed to the nearest whole percent for each nutrient declared in the column described in paragraph (d)(7)(i) of this section for which a DRV has been established, except that the percent for protein may be omitted as provided in paragraph (c)(7) of this section. The percent shall be calculated by dividing either the amount declared on the label for each nutrient or the actual amount of each nutrient (i.e., before rounding) by the DRV for the nutrient, except that the percent for protein shall be calculated as specified in paragraph (c)(7)(ii) of this section. The numerical value shall be followed by the symbol for percent (i.e., %).
(8) Nutrient information for vitamins and minerals (except sodium) shall be separated from information on other nutrients by a bar and may be arrayed vertically as shown in paragraph (d)(12) of this section (e.g., Vitamin D 2 mcg 10%, Calcium 260 mg 20%, Iron 8 mg 45%, Potassium 235 mg 6%) or may be listed horizontally. When listed horizontally in two columns, vitamin D and calcium should be listed on the first line and iron and potassium should be listed on the second line, as shown in paragraph (d)(12) of this section in the side-by-side display. When more than four vitamins and minerals are declared voluntarily as shown in paragraph (d)(12) of this section in the label which illustrates the mandatory plus voluntary provisions of paragraph (d) of this section, they may be declared vertically with percentages listed under the column headed "% Daily Value."
(9) A footnote, preceded by an asterisk, shall be placed beneath the list of vitamins and minerals and shall be separated from the list by a bar, except that the footnote may be omitted from foods that can use the terms "calorie free," "free of calories," "without calories," "trivial source of calories," "negligible source of calories," or "dietary insignificant source of calories" on the label or in the labeling of foods as defined in § 101.60(b). The first sentence of the footnote: "The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet" may be used on foods that can use the terms "calorie free," "free of calories," "without calories," "trivial source of calories," "negligible source of calories," or "dietary insignificant source of calories" on the label or in the labeling of foods as defined in § 101.60(b). The footnote shall state: "*The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice." If the food product is represented or purported to be for children 1 through 3 years of age, the second sentence of the footnote shall substitute "1,000 calories" for "2,000 calories."
(10) Caloric conversion information on a per gram basis for fat, carbohydrate, and protein may be presented beneath the information required in paragraph (d)(9) of this section, separated from that information by a hairline. This information may be presented horizontally as shown in paragraph (d)(12) of this section (i.e., "Calories per gram: fat 9, carbohydrate 4, protein 4") or vertically in columns.
(11)(i) If the space beneath the information on vitamins and minerals is not adequate to accommodate the information required in paragraph (d)(9) of this section, the information required in paragraph (d)(9) may be moved to the right of the column required in paragraph (d)(7)(ii) of this section and set off by a line that distinguishes it and sets it apart from the percent Daily Value information. The caloric conversion information provided for in paragraph (d)(10) of this section may be presented beneath either side or along the full length of the nutrition label.
(ii) If the space beneath the mandatory declaration of potassium is not adequate to accommodate any remaining vitamins and minerals to be declared or the information required in paragraph (d)(9) of this section, the remaining information may be moved to the right and set off by a line that distinguishes it and sets it apart from the nutrients and the percent DV information given to the left. The caloric conversion information provided for in paragraph (d)(10) of this section may be presented beneath either side or along the full length of the nutrition label.
(iii) If there is not sufficient continuous vertical space (i.e., approximately 3 in) to accommodate the required components of the nutrition label up to and including the mandatory declaration of potassium, the nutrition label may be presented in a tabular display as shown in the following sample label.

(12) The following sample labels illustrate the mandatory provisions and mandatory plus voluntary provisions of paragraph (d) of this section and the side-by-side display.
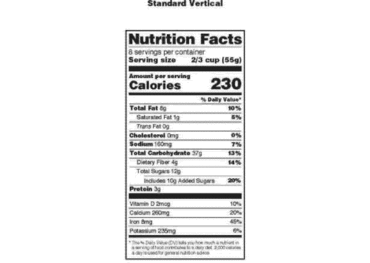
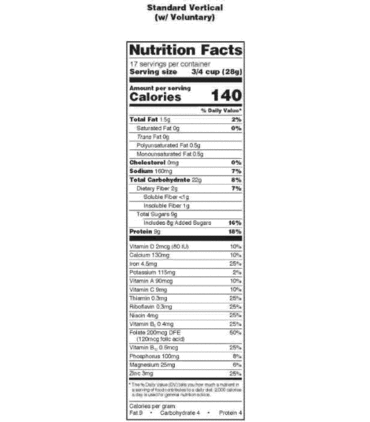

(13)(i) Nutrition labels on the outer label of packages of products that contain two or more separately packaged foods that are intended to be eaten individually (e.g., variety packs of cereals or snack foods) or of packages that are used interchangeably for the same type of food (e.g., round ice cream containers) may use an aggregate display.
(ii) Aggregate displays shall comply with the format requirements of paragraph (d) of this section to the maximum extent possible, except that the identity of each food shall be specified immediately to the right of the "Nutrition Facts" heading, and both the quantitative amount by weight (i.e., g/mg/mcg amounts) and the percent Daily Value for each nutrient shall be listed in separate columns under the name of each food. The following sample label illustrates an aggregate display.
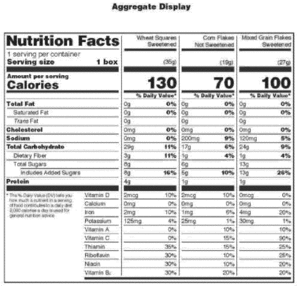
(14) In accordance with § 101.15(c)(2), when nutrition labeling must appear in a second language, the nutrition information may be presented in a separate nutrition label for each language or in one nutrition label with the information in the second language following that in English. Numeric characters that are identical in both languages need not be repeated (e.g., "Protein/Proteinas 2 g"). All required information must be included in both languages.
(e) Nutrition information may be presented for two or more forms of the same food (e.g., both "as purchased" and "as prepared") or for common combinations of food as provided for in paragraph (h)(4) of this section, for different units (e.g., slices of bread or per 100 grams) as provided for in paragraph (b) of this section, or for two or more groups for which RDIs are established (e.g., both infants through 12 months of age and children 1 through 3 years of age) as shown in paragraph (e)(5) of this section. When such dual labeling is provided, equal prominence shall be given to both sets of values. Information shall be presented in a format consistent with paragraph (d) of this section, except that:
(1) Following the serving size information there shall be two or more column headings accurately describing the amount per serving size of the form of the same food (e.g., "Per
1/4 cup mix" and "Per prepared portion"), the combinations of food, the units, or the RDI groups that are being declared as shown in paragraph (e)(5) of this section.
(2) The quantitative information by weight as required in paragraph (d)(7)(i) and the information required in paragraph (d)(7)(ii) of this section shall be presented for the form of the product as packaged and for any other form of the product (e.g., "as prepared" or combined with another ingredient as shown in paragraph (e)(5) of this section).
(3) When the dual labeling is presented for two or more forms of the same food, for combinations of food, for different units, or for two or more groups for which RDIs are established, the quantitative information by weight and the percent Daily Value shall be presented in two columns and the columns shall be separated by vertical lines as shown in paragraph (e)(5) of this section.
(4) Nutrient information for vitamins and minerals (except sodium) shall be separated from information on other nutrients by a bar and shall be arrayed vertically in the following order: Vitamin D, calcium, iron, potassium as shown in paragraph (e)(5) of this section.
(5) The following sample label illustrates the provisions of paragraph (e) of this section:
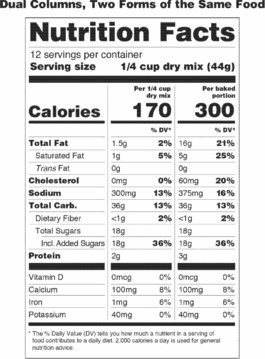
(6) When dual labeling is presented for a food on a per serving basis and per container basis as required in paragraph (b)(12)(i) of this section or on a per serving basis and per unit basis as required in paragraph (b)(2)(i)(D) of this section, the quantitative information by weight as required in paragraph (d)(7)(i) and the percent Daily Value as required in paragraph (d)(7)(ii) shall be presented in two columns, and the columns shall be separated by vertical lines as shown in the displays in paragraph (e)(6)(i) of this section.
(i) Nutrient information for vitamins and minerals shall be separated from information on other nutrients by a bar and shall be arrayed vertically in the following order: Vitamin D, calcium, iron, and potassium as shown in the following sample labels.
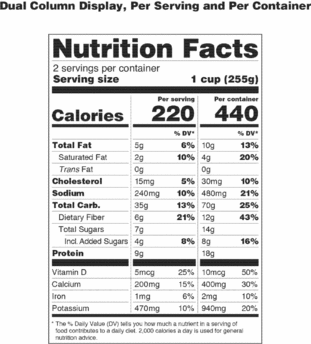
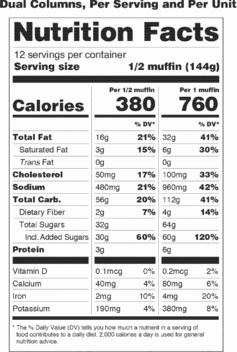
(ii) The following sample label illustrates the provisions of paragraphs (b)(2)(i)(D) and (b)(12)(i) of this section for labels that use the tabular display.
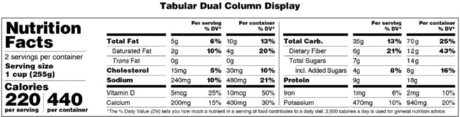
(f) The declaration of nutrition information may be presented in the simplified format set forth herein when a food product contains insignificant amounts of eight or more of the following: Calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, and potassium; except that for foods intended for infants through 12 months of age and children 1 through 3 years of age to which paragraph (j)(5)(i) of this section applies, nutrition information may be presented in the simplified format when a food product contains insignificant amounts of six or more of the following: Calories, total fat, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, and potassium.
(1) An "insignificant amount" shall be defined as that amount that allows a declaration of zero in nutrition labeling, except that for total carbohydrate, dietary fiber, and protein, it shall be an amount that allows a declaration of "less than 1 gram."
(2) The simplified format shall include information on the following nutrients:
(i) Total calories, total fat, total carbohydrate, protein, and sodium;
(ii) Any other nutrients identified in paragraph (f) of this section that are present in the food in more than insignificant amounts; and
(iii) Any vitamins and minerals listed in paragraph (c)(8)(iv) of this section when they are required to be added as a nutrient supplement to foods for which a standard of identity exists.
(iv) Any vitamins or minerals listed in paragraph (c)(8)(iv) of this section voluntarily added to the food as nutrient supplements.
(3) Other nutrients that are naturally present in the food in more than insignificant amounts may be voluntarily declared as part of the simplified format.
(4) If any nutrients are declared as provided in paragraphs (f)(2)(iii), (f)(2)(iv), or (f)(3) of this section as part of the simplified format or if any nutrition claims are made on the label or in labeling, the statement "Not a significant source of ________" (with the blank filled in with the name(s) of any nutrient(s) identified in paragraph (f) of this section that are present in insignificant amounts) shall be included at the bottom of the nutrition label.
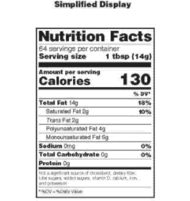
(5) Except as provided for in paragraphs (j)(5) and (j)(13) of this section, nutrient information declared in the simplified format shall be presented in the same manner as specified in paragraphs (d) or (e) of this section, except that the footnote required in paragraph (d)(9) of this section is not required, and an asterisk shall be placed at the bottom of the label followed by the statement "% DV = % Daily Value" when "Daily Value" is not spelled out in the heading, as shown in paragraph (f)(4).
(g) Compliance with this section shall be determined as follows:
(1) A collection of primary containers or units of the same size, type, and style produced under conditions as nearly uniform as possible, designated by a common container code or marking, or in the absence of any common container code or marking, a day's production, constitutes a "lot."
(2) The sample for nutrient analysis shall consist of a composite of 12 subsamples (consumer units), taken 1 from each of 12 different randomly chosen shipping cases, to be representative of a lot. Unless a particular method of analysis is specified in paragraph (c) of this section, composites shall be analyzed by appropriate methods as given in the "Official Methods of Analysis of the AOAC International," or, if no AOAC method is available or appropriate, by other reliable and appropriate analytical procedures.
(3) Two classes of nutrients are defined for purposes of compliance:
(i) Class I. Added nutrients in fortified or fabricated foods; and
(ii) Class II. Naturally occurring (indigenous) nutrients. When a nutrient is naturally occurring (indigenous) in a food or an ingredient that is added to a food, the total amount of such nutrient in the final food product is subject to class II requirements, except that when an exogenous source of the nutrient is also added to the final food product, the total amount of the nutrient in the final food product (indigenous and exogenous) is subject to class I requirements.
(4) A food with a label declaration of a vitamin, mineral, protein, total carbohydrate, dietary fiber, soluble fiber, insoluble fiber, polyunsaturated or monounsaturated fat shall be deemed to be misbranded under section 403(a) of the Federal Food, Drug, and Cosmetic Act (the act) unless it meets the following requirements:
(i) When a vitamin, mineral, protein, or dietary fiber meets the definition of a Class I nutrient, the nutrient content of the composite must be formulated to be at least equal to the value for that nutrient declared on the label.
(ii) When a vitamin, mineral, protein, total carbohydrate, polyunsaturated or monounsaturated fat, or dietary fiber meets the definition of a Class II nutrient, the nutrient content of the composite must be at least equal to 80 percent of the value for that nutrient declared on the label. Provided, That no regulatory action will be based on a determination of a nutrient value that falls below this level by a factor less than the variability generally recognized for the analytical method used in that food at the level involved.
(5) A food with a label declaration of calories, total sugars, added sugars (when the only source of sugars in the food is added sugars), total fat, saturated fat, trans fat, cholesterol, or sodium shall be deemed to be misbranded under section 403(a) of the act if the nutrient content of the composite is greater than 20 percent in excess of the value for that nutrient declared on the label. Provided, That no regulatory action will be based on a determination of a nutrient value that falls above this level by a factor less than the variability generally recognized for the analytical method used in that food at the level involved.
(6) Reasonable excesses of vitamins, minerals, protein, total carbohydrate, dietary fiber, soluble fiber, insoluble fiber, sugar alcohols, polyunsaturated or monounsaturated fat over labeled amounts are acceptable within current good manufacturing practice. Reasonable deficiencies of calories, total sugars, added sugars, total fat, saturated fat, trans fat, cholesterol, or sodium under labeled amounts are acceptable within current good manufacturing practice.
(7) Compliance will be based on the metric measure specified in the label statement of serving size.
(8) Alternatively, compliance with the provisions set forth in paragraphs (g)(1) through (6) of this section may be provided by use of an FDA approved database that has been computed following FDA guideline procedures and where food samples have been handled in accordance with current good manufacturing practice to prevent nutrition loss. FDA approval of a database shall not be considered granted until the Center for Food Safety and Applied Nutrition has agreed to all aspects of the database in writing. The approval will be granted where a clear need is presented (e.g., raw produce and seafood). Approvals will be in effect for a limited time, e.g., 10 years, and will be eligible for renewal in the absence of significant changes in agricultural or industry practices. Approval requests shall be submitted in accordance with the provisions of § 10.30 of this chapter. Guidance in the use of databases may be found in the "FDA Nutrition Labeling Manual - A Guide for Developing and Using Data Bases," available from the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740 or by going to http://www.fda.gov .
(9) When it is not technologically feasible, or some other circumstance makes it impracticable, for firms to comply with the requirements of this section (e.g., to develop adequate nutrient profiles to comply with the requirements of paragraph (c) of this section), FDA may permit alternative means of compliance or additional exemptions to deal with the situation. Firms in need of such special allowances shall make their request in writing to the Center for Food Safety and Applied Nutrition (HFS-800), Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740.
(10) The manufacturer must make and keep written records (e.g., analyses of databases, recipes, formulations, information from recipes or formulations, or batch records) to verify the declared amount of that nutrient on the Nutrition Facts label as follows:
(i) When a mixture of dietary fiber, and added non-digestible carbohydrate(s) that does not meet the definition of dietary fiber, is present in the food, a manufacturer must make and keep written records of the amount of non-digestible carbohydrate(s) added to the food that does not meet the definition of dietary fiber.
(ii) When a mixture of soluble fiber and added non-digestible carbohydrate(s) that does not meet the definition of dietary fiber is present in the food, a manufacturer must make and keep written records necessary to verify the amount of the non-digestible carbohydrate(s) added to the food that does not meet the definition of dietary fiber.
(iii) When a mixture of insoluble fiber and added non-digestible carbohydrate(s) that does not meet the definition of dietary fiber is present in the food, a manufacturer must make and keep written records necessary to verify the amount of the non-digestible carbohydrate(s) added to the food that does not meet the definition of dietary fiber.
(iv) When a mixture of naturally occurring and added sugars is present in the food, a manufacturer must make and keep written records of the amount of added sugars added to the food during the processing of the food, and if packaged as a separate ingredient, as packaged (whether as part of a package containing one or more ingredients or packaged as a single ingredient).
(v) When the amount of sugars added to food products is reduced through non-enzymatic browning and/or fermentation, manufacturers must:
(A) Make and keep records of all relevant scientific data and information relied upon by the manufacturer that demonstrates the amount of added sugars in the food after non-enzymatic browning and/or fermentation and a narrative explaining why the data and information are sufficient to demonstrate the amount of added sugars declared in the finished food, provided the data and information used is specific to the type of food that is subject to non-enzymatic browning and/or fermentation; or
(B) Make and keep records of the amount of added sugars added to the food before and during the processing of the food, and if packaged as a separate ingredient, as packaged (whether as part of a package containing one or more ingredients or packaged as a single ingredient) and in no event shall the amount of added sugars declared exceed the amount of total sugars on the label; or
(C) Submit a petition, under 21 CFR 10.30, to request an alternative means of compliance. The petition must provide scientific data or other information for why the amount of added sugars in a serving of the product is likely to have a significant reduction in added sugars compared to the amount added prior to non-enzymatic browning and/or fermentation. A significant reduction would be where reduction in added sugars after non-enzymatic browning and/or fermentation may be significant enough to impact the label declaration for added sugars by an amount that exceeds the reasonable deficiency acceptable within good manufacturing practice under paragraph (g)(6) of this section. In addition, the scientific data or other information must include the reason that the manufacturer is unable to determine a reasonable approximation of the amount of added sugars in a serving of their finished product and a description of the process that they used to come to that conclusion.
(vi) When a mixture of all rac -[alpha]-tocopherol and RRR-[alpha]-tocopherol is present in a food, manufacturers must make and keep written records of the amount of all rac -[alpha]-tocopherol added to the food and RRR-[alpha]-tocopherol in the finished food.
(vii) When a mixture of folate and folic acid is present in a food, manufacturers must make and keep written records of the amount of synthetic folate and/or folic acid added to the food and the amount of naturally-occurring folate in the finished food.
(11) Records necessary to verify certain nutrient declarations that are specified in paragraph (g)(10) of this section must be kept for a period of at least 2 years after introduction or delivery for introduction of the food into interstate commerce. Such records must be provided to FDA upon request, during an inspection, for official review and photocopying or other means of reproduction. Records required to verify information on the label may be kept either as original records, true copies (such as photocopies, pictures, scanned copies, microfilm, microfiche, or other accurate reproductions of the original records), or electronic records which must be kept in accordance with part 11 of this chapter. These records must be accurate, indelible, and legible.
Failure to make and keep the records or provide the records to appropriate regulatory authorities, as required by this paragraph (g)(11), would result in the food being misbranded under section 403(a)(1) of the act.
(h) Products with separately packaged ingredients or foods, with assortments of food, or to which other ingredients are added by the user may be labeled as follows:
(1) If a product consists of two or more separately packaged ingredients enclosed in an outer container or of assortments of the same type of food (e.g., assorted nuts or candy mixtures) in the same retail package, nutrition labeling shall be located on the outer container or retail package (as the case may be) to provide information for the consumer at the point of purchase. However, when two or more food products are simply combined together in such a manner that no outer container is used, or no outer label is available, each product shall have its own nutrition information, e.g., two boxes taped together or two cans combined in a clear plastic overwrap. When separately packaged ingredients or assortments of the same type of food are intended to be eaten at the same time, the nutrition information may be specified per serving for each component or as a composite value.
(2) If a product consists of two or more separately packaged foods that are intended to be eaten individually and that are enclosed in an outer container (e.g., variety packs of cereals or snack foods), the nutrition information shall:
(i) Be specified per serving for each food in a location that is clearly visible to the consumer at the point of purchase; and
(ii) Be presented in separate nutrition labels or in one aggregate nutrition label with separate columns for the quantitative amount by weight and the percent Daily Value for each food.
(3) If a package contains a variety of foods, or an assortment of foods, and is in a form intended to be used as a gift, the nutrition labeling shall be in the form required by paragraphs (a) through (f) of this section, but it may be modified as follows:
(i) Nutrition information may be presented on the label of the outer package or in labeling within or attached to the outer package.
(ii) In the absence of a reference amount customarily consumed in § 101.12(b) that is appropriate for the variety or assortment of foods in a gift package, the following may be used as the standard serving size for purposes of nutrition labeling of foods subject to this paragraph: 1 ounce for solid foods; 2 fluid ounces for nonbeverage liquids (e.g., syrups); 8 ounces for beverages that consist of milk and fruit juices, nectars and fruit drinks; and 12 fluid ounces for other beverages. However, the reference amounts customarily consumed in § 101.12(b) shall be used for purposes of evaluating whether individual foods in a gift package qualify for nutrient content claims or health claims.
(iii) The number of servings per container may be stated as "varied."
(iv) Nutrition information may be provided per serving for individual foods in the package, or, alternatively, as a composite per serving for reasonable categories of foods in the package having similar dietary uses and similar significant nutritional characteristics. Reasonable categories of foods may be used only if accepted by FDA. In determining whether a proposed category is reasonable, FDA will consider whether the values of the characterizing nutrients in the foods proposed to be in the category meet the compliance criteria set forth in paragraphs (g)(3) through (6) of this section. Proposals for such categories may be submitted in writing to the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740.
(v) If a food subject to paragraph (j)(13) of this section because of its small size is contained in a gift package, the food need not be included in the determination of nutrition information under paragraph (h) of this section if it is not specifically listed in a promotional catalogue as being present in the gift package, and:
(A) It is used in small quantities primarily to enhance the appearance of the gift package; or
(B) It is included in the gift package as a free gift or promotional item.
(4) If a food is commonly combined with other ingredients or is cooked or otherwise prepared before eating, and directions for such combination or preparations are provided, another column of figures may be used to declare nutrition information on the basis of the food as consumed in the format required in paragraph (e) of this section; e.g., a dry ready-to-eat cereal may be described with the percent Daily Value and the quantitative amounts for the cereal as sold (e.g., per ounce), and the percent Daily Value and the quantitative amounts for the cereal and milk as suggested in the label (e.g., per ounce of cereal and
1/2cup of vitamin D fortified skim milk); and a cake mix may be labeled with the percent Daily Value and the quantitative amounts for the dry mix (per serving) and the percent Daily Value and the quantitative amounts for the serving of the final cake when prepared, as shown in paragraph (e)(5) of this section: Provided, that, the type and quantity of the other ingredients to be added to the product by the user and the specific method of cooking and other preparation shall be specified prominently on the label.
(i) Except as provided in paragraphs (j)(13) and (j)(17) of this section, the location of nutrition information on a label shall be in compliance with § 101.2.
(j) The following foods are exempt from this section or are subject to special labeling requirements:
(1)(i) Food offered for sale by a person who makes direct sales to consumers (e.g., a retailer) who has annual gross sales made or business done in sales to consumers that is not more than $500,000 or has annual gross sales made or business done in sales of food to consumers of not more than $50,000, Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information subject the food to the provisions of this section, § 101.10, or § 101.11, as applicable.
(ii) For purposes of this paragraph, calculation of the amount of sales shall be based on the most recent 2-year average of business activity. Where firms have been in business less than 2 years, reasonable estimates must indicate that annual sales will not exceed the amounts specified. For foreign firms that ship foods into the United States, the business activities to be included shall be the total amount of food sales, as well as other sales to consumers, by the firm in the United States.
(2) Except as provided in § 101.11, food products that are:
(i) Served in restaurants, Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information subject the food to the provisions of this section;
(ii) Served in other establishments in which food is served for immediate human consumption (e.g., institutional food service establishments, such as schools, hospitals, and cafeterias; transportation carriers, such as trains and airplanes; bakeries, delicatessens, and retail confectionery stores where there are facilities for immediate consumption on the premises; food service vendors, such as lunch wagons, ice cream shops, mall cookie counters, vending machines, and sidewalk carts where foods are generally consumed immediately where purchased or while the consumer is walking away, including similar foods sold from convenience stores; and food delivery systems or establishments where ready-to-eat foods are delivered to homes or offices), Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising, except as provided in § 101.8(c). Claims or other nutrition information, except as provided in § 101.8(c), subject the food to the provisions of this section;
(iii) Sold only in such facilities, Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information subject the food to the provisions of this section;
(iv) Used only in such facilities and not served to the consumer in the package in which they are received (e.g., foods that are not packaged in individual serving containers); or
(v) Sold by a distributor who principally sells food to such facilities: Provided, That:
(A) This exemption shall not be available for those foods that are manufactured, processed, or repackaged by that distributor for sale to any persons other than restaurants or other establishments that serve food for immediate human consumption, and
(B) The manufacturer of such products is responsible for providing the nutrition information on the products if there is a reasonable possibility that the product will be purchased directly by consumers.
(3) Except as provided in § 101.11, food products that are:
(i) Of the type of food described in paragraphs (j)(2)(i) and (j)(2)(ii) of this section,
(ii) Ready for human consumption,
(iii) Offered for sale to consumers but not for immediate human consumption,
(iv) Processed and prepared primarily in a retail establishment, and
(v) Not offered for sale outside of that establishment (e.g., ready-to-eat foods that are processed and prepared on-site and sold by independent delicatessens, bakeries, or retail confectionery stores where there are no facilities for immediate human consumption; by in-store delicatessen, bakery, or candy departments; or at self-service food bars such as salad bars), Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information subject the food to the provisions of this section.
(4) Except as provided in § 101.11, foods that contain insignificant amounts of all of the nutrients and food components required to be included in the declaration of nutrition information under paragraph (c) of this section, Provided, That the food bears no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information, except as provided in § 101.8(c), subject the food to the provisions of this section. An insignificant amount of a nutrient or food component shall be that amount that allows a declaration of zero in nutrition labeling, except that for total carbohydrate, dietary fiber, and protein, it shall be an amount that allows a declaration of "less than 1 gram." Examples of foods that are exempt under this paragraph include coffee beans (whole or ground), tea leaves, plain unsweetened instant coffee and tea, condiment-type dehydrated vegetables, flavor extracts, and food colors.
(5)(i) Foods, other than infant formula, represented or purported to be specifically for infants through 12 months of age and children 1 through 3 years of age shall bear nutrition labeling. The nutrients declared for infants through 12 months of age and children 1 through 3 years of age shall include calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrates, dietary fiber, total sugars, added sugars, protein, and the following vitamins and minerals: Vitamin D, calcium, iron, and potassium.
(ii) Foods, other than infant formula, represented or purported to be specifically for infants through 12 months of age shall bear nutrition labeling, except that:
(A) Such labeling shall not declare a percent Daily Value for saturated fat, trans fat, cholesterol, sodium, dietary fiber, total sugars, or added sugars and shall not include a footnote.
(B) The following sample label illustrates the provisions of paragraph (j)(5)(ii) of this section.

(C)-(E) [Reserved]
(iii) Foods, other than infant formula, represented or purported to be specifically for children 1 through 3 years of age shall include a footnote that states: "*The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 1,000 calories a day is used for general nutrition advice."
(A) The following sample label illustrates the provisions of paragraph (j)(5)(iii) of this section.
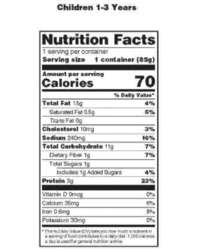
(B) [Reserved]
(6) Dietary supplements, except that such foods shall be labeled in compliance with § 101.36.
(7) Infant formula subject to section 412 of the act, as amended, except that such foods shall be labeled in compliance with part 107 of this chapter.
(8) Medical foods as defined in section 5(b) of the Orphan Drug Act (21 U.S.C. 360ee(b)(3)). A medical food is a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. A food is subject to this exemption only if:
(i) It is a specially formulated and processed product (as opposed to a naturally occurring foodstuff used in its natural state) for the partial or exclusive feeding of a patient by means of oral intake or enteral feeding by tube;
(ii) It is intended for the dietary management of a patient who, because of therapeutic or chronic medical needs, has limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, or who has other special medically determined nutrient requirements, the dietary management of which cannot be achieved by the modification of the normal diet alone;
(iii) It provides nutritional support specifically modified for the management of the unique nutrient needs that result from the specific disease or condition, as determined by medical evaluation;
(iv) It is intended to be used under medical supervision; and
(v) It is intended only for a patient receiving active and ongoing medical supervision wherein the patient requires medical care on a recurring basis for, among other things, instructions on the use of the medical food.
(9) Food products shipped in bulk form that are not for distribution to consumers in such form and that are for use solely in the manufacture of other foods or that are to be processed, labeled, or repacked at a site other than where originally processed or packed.
(10) Raw fruits, vegetables, and fish subject to section 403(q)(4) of the act, except that the labeling of such foods should adhere to guidelines in § 101.45. This exemption is contingent on the food bearing no nutrition claims or other nutrition information in any context on the label or in labeling or advertising. Claims or other nutrition information subject the food to nutrition labeling in accordance with § 101.45. The term fish includes freshwater or marine fin fish, crustaceans, and mollusks, including shellfish, amphibians, and other forms of aquatic animal life.
(11) Packaged single-ingredient products that consist of fish or game meat (i.e., animal products not covered under the Federal Meat Inspection Act or the Poultry Products Inspection Act, such as flesh products from deer, bison, rabbit, quail, wild turkey, or ostrich) subject to this section may provide required nutrition information for a 3-ounce cooked edible portion (i.e., on an "as prepared" basis), except that:
(i) Such products that make claims that are based on values as packaged must provide nutrition information on an as packaged basis, and
(ii) Nutrition information is not required for custom processed fish or game meats.
(12) Game meats (i.e., animal products not covered under the Federal Meat Inspection Act or the Poultry Products Inspection Act, such as flesh products from deer, bison, rabbit, quail, wild turkey, or ostrich) may provide required nutrition information on labeling in accordance with the provisions of paragraph (a)(2) of this section.
(13)(i) Foods in small packages that have a total surface area available to bear labeling of less than 12 square inches, Provided, That the labels for these foods bear no nutrition claims or other nutrition information in any context on the label or in labeling or advertising, except as provided in § 101.8(c). Claims or other nutrition information, except as provided in § 101.8(c), subject the food to the provisions of this section. Foods in packages subject to requirements of paragraphs (j)(13)(ii)(A)(1 ) and (2 ) of this section do not require the information in paragraphs (d)(9) and (f)(5) related to the footnote, however the abbreviated footnote statement "% DV = % Daily Value" may be used.
(A) The manufacturer, packer, or distributor shall provide on the label of packages that qualify for and use this exemption an address or telephone number that a consumer can use to obtain the required nutrition information (e.g., "For nutrition information, call 1-800-123-4567").
(B) When such products bear nutrition labeling, either voluntarily or because nutrition claims or other nutrition information is provided, all required information shall be in type size no smaller than 6 point or all upper-case type of
1/16 inches minimum height, except that individual serving-size packages of food served with meals in restaurants, institutions, and on board passenger carriers, and not intended for sale at retail, may comply with § 101.2(c)(2).
(ii) Foods in packages that have a total surface area available to bear labeling of 40 or less square inches may modify the requirements of paragraphs (c) through (f) and (i) of this section by one or more of the following means:
(A) Presenting the required nutrition information in a tabular or, as provided below, linear (i.e., string) fashion rather than in vertical columns if the product has a total surface area available to bear labeling of less than 12 square inches, or if the product has a total surface area available to bear labeling of 40 or less square inches and the package shape or size cannot accommodate a standard vertical column or tabular display on any label panel. Nutrition information may be given in a linear fashion only if the label will not accommodate a tabular display.
(1 ) The following sample label illustrates the tabular display for small packages.

(2 ) The following sample label illustrates the linear display.

(B) Using any of the following abbreviations:
Serving size - Serv size
Servings per container - Servings
Calories from saturated fat - Sat fat cal
Saturated fat - Sat fat
Monounsaturated fat - Monounsat fat
Polyunsaturated fat - Polyunsat fat
Cholesterol - Cholest
Total carbohydrate - Total carb. This abbreviation can also be used on dual-column displays as shown in paragraphs (e)(5), (e)(6)(i), and (e)(6)(ii) of this section.
Dietary fiber - Fiber
Soluble fiber - Sol fiber
Insoluble fiber - Insol fiber
Sugar alcohol - Sugar alc
Vitamin - Vit. This abbreviation can also be used on the standard vertical side-by-side display as shown in paragraph (d)(12) of this section.
Potassium - Potas. This abbreviation can also be used on the standard vertical side-by-side display as shown in paragraph (d)(12) of this section.
Includes - Incl. This abbreviation can also be used on dual-column displays as shown in paragraphs (e)(5), (e)(6)(i), and (e)(6)(ii) of this section.
(C) Presenting the required nutrition information on any label panel.
(14) Shell eggs packaged in a carton that has a top lid designed to conform to the shape of the eggs are exempt from outer carton label requirements where the required nutrition information is clearly presented immediately beneath the carton lid or in an insert that can be clearly seen when the carton is opened.
(15) The unit containers in a multiunit retail food package where:
(i) The multiunit retail food package labeling contains all nutrition information in accordance with the requirements of this section;
(ii) The unit containers are securely enclosed within and not intended to be separated from the retail package under conditions of retail sale; and
(iii) Each unit container is labeled with the statement "This Unit Not Labeled For Retail Sale" in type size not less than 1/16-inch in height, except that this statement shall not be required when the inner unit containers bear no labeling at all. The word "individual" may be used in lieu of or immediately preceding the word "Retail" in the statement.
(16) Food products sold from bulk containers: Provided, That nutrition information required by this section be displayed to consumers either on the labeling of the bulk container plainly in view or in accordance with the provisions of paragraph (a)(2) of this section.
(17) Foods in packages that have a total surface area available to bear labeling greater than 40 square inches but whose principal display panel and information panel do not provide sufficient space to accommodate all required information may use any alternate panel that can be readily seen by consumers for the nutrition label. The space needed for vignettes, designs, and other nonmandatory label information on the principal display panel may be considered in determining the sufficiency of available space on the principal display panel for the placement of the nutrition label. Nonmandatory label information on the information panel shall not be considered in determining the sufficiency of available space for the placement of the nutrition label.
(18) Food products that are low-volume (that is, they meet the requirements for units sold in paragraphs (j)(18)(i) or (j)(18)(ii) of this section); that, except as provided in paragraph (j)(18)(iv) of this section, are the subject of a claim for an exemption that provides the information required under paragraph (j)(18)(iv) of this section, that is filed before the beginning of the time period for which the exemption is claimed, and that is filed by a person, whether it is the manufacturer, packer, or distributor, that qualifies to claim the exemption under the requirements for average full-time equivalent employees in paragraphs (j)(18)(i) or (j)(18)(ii) of this section; and whose labels, labeling, and advertising do not provide nutrition information or make a nutrient content or health claim.
(i) For food products first introduced into interstate commerce before May 8, 1994, the product shall be exempt for the period:
(A) Between May 8, 1995, and May 7, 1996, if, for the period between May 8, 1994, and May 7, 1995, the person claiming the exemption employed fewer than an average of 300 full-time equivalent employees and fewer than 400,000 units of that product were sold in the United States; and
(B) Between May 8, 1996, and May 7, 1997, if for the period between May 8, 1995, and May 7, 1996, the person claiming the exemption employed fewer than an average of 200 full-time equivalent employees and fewer than 200,000 units of that product were sold in the United States.
(ii) For all other food products, the product shall be eligible for an exemption for any 12-month period if, for the preceding 12 months, the person claiming the exemption employed fewer than an average of 100 full-time equivalent employees and fewer than 100,000 units of that product were sold in the United States, or in the case of a food product that was not sold in the 12-month period preceding the period for which exemption is claimed, fewer than 100,000 units of such product are reasonably anticipated to be sold in the United States during the period for which exemption is claimed.
(iii) If a person claims an exemption under paragraphs (j)(18)(i) or (j)(18)(ii) of this section for a food product and then, during the period of such exemption, the number of full-time equivalent employees of such person exceeds the appropriate number, or the number of food products sold in the United States exceeds the appropriate number, or, if at the end of the period of such exemption, the food product no longer qualifies for an exemption under the provisions of paragraphs (j)(18)(i) or (j)(18)(ii) of this section, such person shall have 18 months from the date that the product was no longer qualified as a low-volume product of a small business to comply with this section.
(iv) A notice shall be filed with the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740 and contain the following information, except that if the person is not an importer and has fewer than 10 full-time equivalent employees, that person does not have to file a notice for any food product with annual sales of fewer than 10,000 total units:
(A) Name and address of person requesting exemption. This should include a telephone number or FAX number that can be used to contact the person along with the name of a specific contact;
(B) Names of the food products (including the various brand names) for which exemption is claimed;
(C) Name and address of the manufacturer, distributor, or importer of the food product for which an exemption is claimed, if different than the person that is claiming the exemption;
(D) The number of full-time equivalent employees. Provide the average number of full-time equivalent individuals employed by the person and its affiliates for the 12 months preceding the period for which a small business exemption is claimed for a product. The average number of full-time equivalent employees is to be determined by dividing the total number of hours of salary or wages paid to employees of the person and its affiliates by the number of hours of work in a year, 2,080 hours (i.e., 40 hours * 52 weeks);
(E) Approximate total number of units of the food product sold by the person in the United States in the 12-month period preceding that for which a small business exemption is claimed. Provide the approximate total number of units sold, or expected to be sold, in a 12-month period for each product for which an exemption is claimed. For products that have been in production for 1 year or more prior to the period for which exemption is claimed, the 12-month period is the period immediately preceding the period for which an exemption is claimed. For other products, the 12-month period is the period for which an exemption is claimed; and
(F) The notice shall be signed by a responsible individual for the person who can certify the accuracy of the information presented in the notice. The individual shall certify that the information contained in the notice is a complete and accurate statement of the average number of full-time equivalent employees of this person and its affiliates and of the number of units of the product for which an exemption is claimed sold by the person. The individual shall also state that should the average number of full-time equivalent employees or the number of units of food products sold in the United States by the person exceed the applicable numbers for the time period for which exemption is claimed, the person will notify FDA of that fact and the date on which the number of employees or the number of products sold exceeded the standard.
(v) FDA may by regulation lower the employee or units of food products requirements of paragraph (j)(18)(ii) of this section for any food product first introduced into interstate commerce after May 8, 2002, if the agency determines that the cost of compliance with such lower requirement will not place an undue burden on persons subject to it.
(vi) For the purposes of this paragraph, the following definitions apply:
(A) Unit means the packaging or, if there is no packaging, the form in which a food product is offered for sale to consumers.
(B) Food product means food in any sized package which is manufactured by a single manufacturer or which bears the same brand name, which bears the same statement of identity, and which has similar preparation methods.
(C) Person means all domestic and foreign affiliates, as defined in 13 CFR 121.401, of the corporation, in the case of a corporation, and all affiliates, as defined in 13 CFR 121.401, of a firm or other entity, when referring to a firm or other entity that is not a corporation.
(D) Full-time equivalent employee means all individuals employed by the person claiming the exemption. This number shall be determined by dividing the total number of hours of salary or wages paid directly to employees of the person and of all of its affiliates by the number of hours of work in a year, 2,080 hours (i.e., 40 hours * 52 weeks).
(k) A food labeled under the provisions of this section shall be deemed to be misbranded under sections 201(n) and 403(a) of the act if its label or labeling represents, suggests, or implies:
(1) That the food, because of the presence or absence of certain dietary properties, is adequate or effective in the prevention, cure, mitigation, or treatment of any disease or symptom. Information about the relationship of a dietary property to a disease or health-related condition may only be provided in conformance with the requirements of § 101.14 and part 101, subpart E.
(2) That the lack of optimum nutritive quality of a food, by reason of the soil on which that food was grown, is or may be responsible for an inadequacy or deficiency in the quality of the daily diet.
(3) That the storage, transportation, processing, or cooking of a food is or may be responsible for an inadequacy or deficiency in the quality of the daily diet.
(4) That a natural vitamin in a food is superior to an added or synthetic vitamin.
(l) The standards required in this section are incorporated by reference into this section with the approval of the Director of the Federal Register under 5 U.S.C. 552(a) and 1 CFR part 51. All approved material is available for inspection at the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-2404 and is available from the sources indicated below. It is also available for inspection at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html .
(1) AOAC Reseller. Techstreet, 6300 Interfirst Dr., Ann Arbor, MI 48108, Toll free in United States: 1-800-699-9277, Outside United States: 1-734-780-8000, Fax: 1-734-780-2046, www.techstreet.com, techstreet.service@thomsonreuters.com . FDA does not endorse any particular reseller and notes that other resellers also may have the reference for sale. Consult FDA at 240-402-2404 for more information on additional resellers.
(i) "Official Methods of Analysis of the AOAC INTERNATIONAL," 19th Edition, Volumes 1 and 2, 2012.
(ii) [Reserved]
(2) Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO), Publications Division, Viale delle Terme di Caracalla, 00100 Rome, Italy
(i) FAO Food and Nutrition Paper 51,"Report of the Joint FAO/WHO Expert Consultation on Protein Quality Evaluation," Rome, 1991. http://apps.who.int/iris/bitstream/10665/38133/1/9251030979_eng.pdf .
(ii) [Reserved]
(3) United States Department of Agriculture (USDA), Agricultural Research Service, Washington, DC, Nutrient Data Laboratory, Bldg. 005 Room 105 BARC-West, Beltsville, MD 20705, 301-504-0630. http://www.ars.usda.gov/News/docs.htm?docid=9447 .
(i) USDA Handbook No. 74, Energy Value of Foods - basis and derivation, by A. L. Merrill and B. K. Watt, (slightly revised, 1973) http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/Classics/ah74.pdf .
(ii) [Reserved]
[58 FR 2175, Jan. 6, 1993] Editorial Note: For Federal Register citations affecting § 101.9, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and at www.govinfo.gov.
|
|
|
Sec. 101.10 Nutrition labeling of restaurant foods whose labels or labeling bear nutrient content claims or health claims.
|
|
Nutrition labeling in accordance with § 101.9 shall be provided upon request for any restaurant food or meal for which a nutrient content claim (as defined in § 101.13 or in subpart D of this part) or a health claim (as defined in § 101.14 and permitted by a regulation in subpart E of this part) is made, except that information on the nutrient amounts that are the basis for the claim (e.g., "low fat, this meal provides less than 10 grams of fat") may serve as the functional equivalent of complete nutrition information as described in § 101.9. For the purposes of this section, restaurant food includes two categories of food. It includes food which is served in restaurants or other establishments in which food is served for immediate human consumption or which is sold for sale or use in such establishments. It also includes food which is processed and prepared primarily in a retail establishment, which is ready for human consumption, which is of the type described in the previous sentence, and which is offered for sale to consumers but not for immediate human consumption in such establishment and which is not offered for sale outside such establishment. For standard menu items that are offered for sale in covered establishments (as defined in § 101.11(a)), the information in the written nutrition information required by § 101.11(b)(2)(ii)(A) will serve to meet the requirements of this section. Nutrient levels may be determined by nutrient databases, cookbooks, or analyses or by other reasonable bases that provide assurance that the food or meal meets the nutrient requirements for the claim. Presentation of nutrition labeling may be in various forms, including those provided in § 101.45 and other reasonable means.
[79 FR 71253, Dec. 1, 2014]
|
|
|
Sec. 101.11 Nutrition labeling of standard menu items in covered establishments.
|
|
(a) Definitions. The definitions of terms in section 201 of the Federal Food, Drug, and Cosmetic Act apply to such terms when used in this section. In addition, for purposes of this section:
Authorized official of a restaurant or similar retail food establishment means the owner, operator, agent in charge, or other person authorized by the owner, operator, or agent in charge to register the restaurant or similar retail food establishment, which is not otherwise subject to section 403(q)(5)(H) of the Federal Food, Drug, and Cosmetic Act, with FDA for the purposes of paragraph (d) of this section.
Combination meal means a standard menu item that consists of more than one food item, for example a meal that includes a sandwich, a side dish, and a drink. A combination meal may be represented on the menu or menu board in narrative form, numerically, or pictorially. Some combination meals may include a variable menu item or be a variable menu item as defined in this paragraph where the components may vary. For example, the side dish may vary among several options (e.g., fries, salad, or onion rings) or the drinks may vary (e.g., soft drinks, milk, or juice) and the customer selects which of these items will be included in the meal.
Covered establishment means a restaurant or similar retail food establishment that is a part of a chain with 20 or more locations doing business under the same name (regardless of the type of ownership, e.g., individual franchises) and offering for sale substantially the same menu items, as well as a restaurant or similar retail food establishment that is registered to be covered under paragraph (d) of this section.
Custom order means a food order that is prepared in a specific manner based on an individual customer's request, which requires the covered establishment to deviate from its usual preparation of a standard menu item, e.g., a club sandwich without the bacon if the establishment usually includes bacon in its club sandwich.
Daily special means a menu item that is prepared and offered for sale on a particular day, that is not routinely listed on a menu or menu board or offered by the covered establishment, and that is promoted by the covered establishment as a special menu item for that particular day.
Doing business under the same name means sharing the same name. The term "name" refers to either:
(i) The name of the establishment presented to the public; or
(ii) If there is no name of the establishment presented to the public (e.g., an establishment with the generic descriptor "concession stand"), the name of the parent entity of the establishment. When the term "name" refers to the name of the establishment presented to the public under paragraph (i) of this definition, the term "same" includes names that are slight variations of each other, for example, due to the region, location, or size (e.g., "New York Ave. Burgers" and "Pennsylvania Ave. Burgers" or "ABC" and "ABC Express").
Food on display means restaurant-type food that is visible to the customer before the customer makes a selection, so long as there is not an ordinary expectation of further preparation by the consumer before consumption.
Food that is part of a customary market test means food that appears on a menu or menu board for less than 90 consecutive days in order to test consumer acceptance of the product.
Location means a fixed position or site.
Menu or menu board means the primary writing of the covered establishment from which a customer makes an order selection, including, but not limited to, breakfast, lunch, and dinner menus; dessert menus; beverage menus; children's menus; other specialty menus; electronic menus; and menus on the Internet. Determining whether a writing is or is part of the primary writing of the covered establishment from which a customer makes an order selection depends on a number of factors, including whether the writing lists the name of a standard menu item (or an image depicting the standard menu item) and the price of the standard menu item, and whether the writing can be used by a customer to make an order selection at the time the customer is viewing the writing. The menus may be in different forms, e.g., booklets, pamphlets, or single sheets of paper. Menu boards include those inside a covered establishment as well as drive-through menu boards at covered establishments.
Offering for sale substantially the same menu items means offering for sale a significant proportion of menu items that use the same general recipe and are prepared in substantially the same way with substantially the same food components, even if the name of the menu item varies, (e.g., "Bay View Crab Cake" and "Ocean View Crab Cake"). "Menu items" in this definition refers to food items that are listed on a menu or menu board or that are offered as self-service food or food on display. Restaurants and similar retail food establishments that are part of a chain can still be offering for sale substantially the same menu items if the availability of some menu items varies within the chain. Having the same name may indicate, but does not necessarily guarantee, that menu items are substantially the same.
Restaurant or similar retail food establishment means a retail establishment that offers for sale restaurant-type food, except if it is a school as defined by 7 CFR 210.2 or 220.2.
Restaurant-type food means food that is:
(i) Usually eaten on the premises, while walking away, or soon after arriving at another location; and
(ii) Either:
(A) Served in restaurants or other establishments in which food is served for immediate human consumption or which is sold for sale or use in such establishments; or
(B) Processed and prepared primarily in a retail establishment, ready for human consumption, of the type described in paragraph (ii)(A) of this definition, and offered for sale to consumers but not for immediate human consumption in such establishment and which is not offered for sale outside such establishment.
Self-service food means restaurant-type food that is available at a salad bar, buffet line, cafeteria line, or similar self-service facility and that is served by the customers themselves. Self-service food also includes self-service beverages.
Standard menu item means a restaurant-type food that is routinely included on a menu or menu board or routinely offered as a self-service food or food on display.
Temporary menu item means a food that appears on a menu or menu board for less than a total of 60 days per calendar year. The 60 days includes the total of consecutive and non-consecutive days the item appears on the menu.
Variable menu item means a standard menu item that comes in different flavors, varieties, or combinations, and is listed as a single menu item.
(b) Requirements for nutrition labeling for food sold in covered establishments - (1) Applicability. (i) The labeling requirements in this paragraph (b) apply to standard menu items offered for sale in covered establishments.
(ii)(A) The labeling requirements in this paragraph (b) do not apply to foods that are not standard menu items, including:
(1 ) Items such as condiments that are for general use, including those placed on the table or on or behind the counter; daily specials; temporary menu items; custom orders; food that is part of a customary market test; and
(2 ) Self-service food and food on display that is offered for sale for less than a total of 60 days per calendar year or fewer than 90 consecutive days in order to test consumer acceptance.
(B) The labeling requirements of paragraph (b)(2)(iii) of this section do not apply to alcoholic beverages that are foods on display and are not self-service foods.
(2) Nutrition information. (i) Except as provided by paragraph (b)(2)(i)(A)(8 ) of this section, the following must be provided on menus and menu boards:
(A) The number of calories contained in each standard menu item listed on the menu or menu board, as usually prepared and offered for sale. In the case of multiple-serving standard menu items, this means the calories declared must be for the whole menu item listed on the menu or menu board as usually prepared and offered for sale (e.g., "pizza pie: 1600 cal"); or per discrete serving unit as long as the discrete serving unit (e.g., pizza slice) and total number of discrete serving units contained in the menu item are declared on the menu or menu board, and the menu item is usually prepared and offered for sale divided in discrete serving units (e.g., "pizza pie: 200 cal/slice, 8 slices"). The calories must be declared in the following manner:
(1 ) The number of calories must be listed adjacent to the name or the price of the associated standard menu item, in a type size no smaller than the type size of the name or the price of the associated standard menu item, whichever is smaller, in the same color, or a color at least as conspicuous as that used for the name of the associated standard menu item, and with the same contrasting background or a background at least as contrasting as that used for the name of the associated standard menu item.
(2 ) To the nearest 5-calorie increment up to and including 50 calories and to the nearest 10-calorie increment above 50 calories, except that amounts less than 5 calories may be expressed as zero.
(3 ) The term "Calories" or "Cal" must appear as a heading above a column listing the number of calories for each standard menu item or adjacent to the number of calories for each standard menu item. If the term "Calories" or "Cal" appears as a heading above a column of calorie declarations, the term must be in a type size no smaller than the smallest type size of the name or price of any menu item on that menu or menu board in the same color or a color at least as conspicuous as that used for that name or price and in the same contrasting background or a background at least as contrasting as that used for that name or price. If the term "Calories" or "Cal" appears adjacent to the number of calories for the standard menu item, the term "Calories" or "Cal" must appear in the same type size and in the same color and contrasting background as the number of calories.
(4 ) Additional requirements that apply to each individual variable menu item:
(i ) When the menu or menu board lists flavors or varieties of an entire individual variable menu item (such as soft drinks, ice cream, doughnuts, dips, and chicken that can be grilled or fried), the calories must be declared separately for each listed flavor or variety. Where flavors or varieties have the same calorie amounts (after rounding in accordance with paragraph (b)(2)(i)(A)(2 ) of this section), the calorie declaration for such flavors or varieties can be listed as a single calorie declaration adjacent to the flavors or varieties, provided that the calorie declaration specifies that the calorie amount listed represents the calorie amounts for each individual flavor or variety.
(ii ) When the menu or menu board does not list flavors or varieties for an entire individual variable menu item, and only includes a general description of the variable menu item (e.g., "soft drinks"), the calories must be declared for each option with a slash between the two calorie declarations where only two options are available (e.g., "150/250 calories") or as a range in accordance with the requirements of paragraph (b)(2)(i)(A)(7) of this section where more than two options are available (e.g., "100-250 calories").
(iii ) When the menu or menu board describes flavors or varieties for only part of an individual variable menu item (such as different types of cheese offered in a grilled cheese sandwich (e.g., "Grilled Cheese (Cheddar or Swiss)"), the calories must be declared for each option with a slash between the two calorie declarations where only two options are available (e.g., "450/500 calories") or as a range in accordance with the requirements of paragraph (b)(2)(i)(A)(7 ) of this section where more than two options are available (e.g., "450-550 calories").
(5 ) Additional requirements that apply to a variable menu item that is offered for sale with the option of adding toppings listed on the menu or menu board. When the menu or menu board lists toppings that can be added to a menu item (such as pizza or ice cream):
(i ) The calories must be declared for the basic preparation of the menu item as listed (e.g., "small pizza pie," "single scoop ice cream").
(ii ) The calories must be separately declared for each topping listed on the menu or menu board (e.g., pepperoni, sausage, green peppers, onions on pizza; fudge, almonds, sprinkles on ice cream), specifying that the calories are added to the calories contained in the basic preparation of the menu item. Where toppings have the same calorie amounts (after rounding in accordance with paragraph (b)(2)(i)(A)(2 ) of this section), the calorie declaration for such toppings can be listed as a single calorie declaration adjacent to the toppings, provided that the calorie declaration specifies that the calorie amount listed represents the calorie amount for each individual topping.
(iii ) The calories for the basic preparation of the menu item must be declared for each size of the menu item. The calories for each topping listed on the menu or menu board must be declared for each size of the menu item, or declared using a slash between the two calorie declarations for each topping where only two sizes of the menu item are available (e.g., "adds 150/250 cal") or as a range for each topping in accordance with the requirements of paragraph (b)(2)(i)(A)(7 ) of this section where more than two sizes of the menu item are available (e.g., "adds 100-250 cal"). If a slash between two calorie declarations or a range of calorie declarations is used, the menu or menu board must indicate that the variation in calories for each topping arises from the size of the menu item to which the toppings are added.
(iv ) If the amount of the topping included on the basic preparation of the menu item decreases based on the total number of toppings ordered for the menu item (such as is sometimes the case with pizza toppings), the calories for each topping must be declared as single values representing the calories for each topping when added to a one-topping menu item, specifying that the calorie declaration is for the topping when added to a one-topping menu item.
(6 ) Additional requirements that apply to a combination meal. Except as provided in paragraph (b)(2)(i)(A)(6 )(iv ) of this section:
(i ) When the menu or menu board lists two options for menu items in a combination meal (e.g., a sandwich with a side salad or chips), the calories must be declared for each option with a slash between the two calorie declarations (e.g., "350/450 calories").
(ii ) When the menu or menu board lists three or more options for menu items in a combination meal (e.g., a sandwich with chips, a side salad, or fruit), the calories must be declared as a range in accordance with the requirements of paragraph (b)(2)(i)(A)(7 ) of this section (e.g., "350-500 calories").
(iii ) When the menu or menu board includes a choice to increase or decrease the size of a combination meal, the calorie difference must be declared for the increased or decreased size with a slash between two calorie declarations (e.g., "Adds 100/150 calories," "Subtracts 100/150 calories") if the menu or menu board lists two options for menu items in the combination meal, or as a range in accordance with the requirements of paragraph (b)(2)(i)(A)(7 ) of this section (e.g., "Adds 100-250 calories," "Subtracts 100-250 calories") if the menu or menu board lists three or more options for menu items in the combination meal.
(iv ) Where the menu or menu board describes an opportunity for a consumer to combine standard menu items for a special price (e.g.," Combine Any Sandwich with Any Soup or Any Salad for $8.99"), and the calories for each standard menu item, including each size option as described in paragraph (b)(2)(i)(A)(6)(iii ) of this section if applicable, available for the consumer to combine are declared elsewhere on the menu or menu board, the requirements of paragraphs (b)(2)(i)(A)(6 )(i ), (ii ), and (iii ) of this section do not apply.
(7 ) Additional format requirements for declaring calories for an individual variable menu item, a combination meal, and toppings as a range, if applicable. Calories declared as a range must be in the format "xx-yy," where "xx" is the caloric content of the lowest calorie variety, flavor, or combination, and "yy" is the caloric content of the highest calorie variety, flavor, or combination.
(8 ) Exception for a variable menu item that has no clearly identifiable upper bound to the range of calories: If the variable menu item appears on the menu or menu board and is a self-service food or food on display, and there is no clearly identifiable upper bound to the range, e.g., all-you-can-eat buffet, then the menu or menu board must include a statement, adjacent to the name or price of the item, referring customers to the self-service facility for calorie information, e.g., "See buffet for calorie declarations." This statement must appear in a type size no smaller than the type size of the name or price of the variable menu item, whichever is smaller, and in the same color or a color at least as conspicuous as that used for that name or price, with the same contrasting background or a background at least as contrasting as that used for that name or price.
(9 ) Additional requirements that apply to beverages that are not self-service. For beverages that are not self-service, calories must be declared based on the full volume of the cup served without ice, unless the covered establishment ordinarily dispenses and offers for sale a standard beverage fill (i.e., a fixed amount that is less than the full volume of the cup per cup size) or dispenses a standard ice fill (i.e., a fixed amount of ice per cup size). If the covered establishment ordinarily dispenses and offers for sale a standard beverage fill or dispenses a standard ice fill, the covered establishment must declare calories based on such standard beverage fill or standard ice fill.
(B) The following statement designed to enable consumers to understand, in the context of a total daily diet, the significance of the calorie information provided on menus and menu boards: "2,000 calories a day is used for general nutrition advice, but calorie needs vary." For menus and menu boards targeted to children, the following options may be used as a substitute for or in addition to the succinct statement: "1,200 to 1,400 calories a day is used for general nutrition advice for children ages 4 to 8 years, but calorie needs vary." or "1,200 to 1,400 calories a day is used for general nutrition advice for children ages 4 to 8 years and 1,400 to 2,000 calories a day for children ages 9 to 13 years, but calorie needs vary."
(1 ) This statement must be posted prominently and in a clear and conspicuous manner in a type size no smaller than the smallest type size of any calorie declaration appearing on the same menu or menu board and in the same color or in a color at least as conspicuous as that used for the calorie declarations and with the same contrasting background or a background at least as contrasting as that used for the calorie declarations.
(2 ) For menus, this statement must appear on the bottom of each page of the menu. On menu pages that also bear the statement required by paragraph (b)(2)(i)(C) of this section, this statement must appear immediately above, below, or beside the statement required by paragraph (b)(2)(i)(C) of this section.
(3 ) For menu boards, this statement must appear on the bottom of the menu board, immediately above, below, or beside the statement required by paragraph (b)(2)(i)(C) of this section.
(C) The following statement regarding the availability of the additional written nutrition information required in paragraph (b)(2)(ii) of this section must be on all forms of the menu or menu board: "Additional nutrition information available upon request."
(1 ) This statement must be posted prominently and in a clear and conspicuous manner in a type size no smaller than the smallest type size of any calorie declaration appearing on the same menu or menu board and in the same color or in a color at least as conspicuous as that used for the caloric declarations, and with the same contrasting background or a background at least as contrasting as that used for the caloric declarations.
(2 ) For menus, the statement must appear on the bottom of the first page with menu items immediately above, below, or beside the succinct statement required by paragraph (b)(2)(i)(B) of this section.
(3 ) For menu boards, the statement must appear on the bottom of the menu board immediately above, below, or beside the succinct statement required by paragraph (b)(2)(i)(B) of this section.
(ii) The following nutrition information for a standard menu item must be available in written form on the premises of the covered establishment and provided to the customer upon request. This nutrition information must be presented in the order listed and using the measurements listed, except as provided in paragraph (b)(2)(ii)(B) of this section. Rounding of these nutrients must be in compliance with § 101.9(c). The information must be presented in a clear and conspicuous manner, including using a color, type size, and contrasting background that render the information likely to be read and understood by the ordinary individual under customary conditions of purchase and use. Covered establishments may use the abbreviations allowed for Nutrition Facts for certain packaged foods in § 101.9(j)(13)(ii)(B):
(A)(1 ) Total calories (cal);
(2 ) Calories from fat (fat cal);
(3 ) Total fat (g);
(4 ) Saturated fat (g);
(5 ) Tran s fat (g);
(6 ) Cholesterol (mg);
(7 ) Sodium (mg);
(8 ) Total carbohydrate (g);
(9 ) Dietary fiber (g);
(10 ) Sugars (g); and
(11 ) Protein (g).
(B) If a standard menu item contains insignificant amounts of all the nutrients required to be disclosed in paragraph (b)(2)(ii)(A) of this section, the establishment is not required to include nutrition information regarding the standard menu item in the written form. However, if the covered establishment makes a nutrient content claim or health claim, the establishment is required to provide nutrition information on the nutrient that is the subject of the claim in accordance with § 101.10. For standard menu items that contain insignificant amounts of six or more of the required nutrients, the declaration of nutrition information required by paragraph (b)(2)(ii)(A) of this section may be presented in a simplified format.
(1 ) An insignificant amount is defined as that amount that allows a declaration of zero in nutrition labeling, except that for total carbohydrates, dietary fiber, and protein, it must be an amount that allows a declaration of "less than one gram."
(2 ) The simplified format must include information, in a column, list, or table, on the following nutrients:
(i ) Total calories, total fat, total carbohydrates, protein, and sodium; and
(ii ) Calories from fat, and any other nutrients identified in paragraph (b)(2)(ii)(A) of this section that are present in more than insignificant amounts.
(3 ) If the simplified format is used, the statement "Not a significant source of ____" (with the blank filled in with the names of the nutrients required to be declared in the written nutrient information and calories from fat that are present in insignificant amounts) must be included at the bottom of the list of nutrients.
(C) For variable menu items, the nutrition information listed in paragraph (b)(2)(ii)(A) of this section must be declared as follows for each size offered for sale:
(1 ) The nutrition information required in paragraph (b)(2)(ii)(A) of this section must be declared for the basic preparation of the item and, separately, for each topping, flavor, or variable component.
(2 ) Additional format requirements for toppings if the amount of the topping included on the basic preparation of the menu item decreases based on the total number of toppings ordered for the menu item (such as is sometimes the case with pizza toppings). The nutrients for such topping must be declared as single values representing the nutrients for each topping when added to a one-topping menu item, specifying that the nutrient declaration is for the topping when added to a one-topping menu item.
(3 ) If the calories and other nutrients are the same for different flavors, varieties, and variable components of the combination meal, each variety, flavor, and variable component of the combination meal is not required to be listed separately. All items that have the same nutrient values could be listed together with the nutrient values listed only once.
(D) The written nutrition information required in paragraph (b)(2)(ii)(A) of this section may be provided on a counter card, sign, poster, handout, booklet, loose leaf binder, or electronic device such as a computer, or in a menu, or in any other form that similarly permits the written declaration of the required nutrient content information for all standard menu items. If the written nutrition information is not in a form that can be given to the customer upon request, it must be readily available in a manner and location on the premises that allows the customer/consumer to review the written nutrition information upon request.
(iii) The following must be provided for a standard menu item that is self-service or on display.
(A) Calories per displayed food item (e.g., a bagel, a slice of pizza, or a muffin), or if the food is not offered for sale in a discrete unit, calories per serving (e.g., scoop, cup), and the serving or discrete unit used to determine the calorie content (e.g., "per scoop" or "per muffin") on either: A sign adjacent to and clearly associated with the corresponding food; (e.g., "150 calories per scoop); a sign attached to a sneeze guard with the calorie declaration and the serving or unit used to determine the calorie content above each specific food so that the consumer can clearly associate the calorie declaration with the food, except that if it is not clear to which food the calorie declaration and serving or unit refers, then the sign must also include the name of the food, e.g., "Broccoli and cheese casserole - 200 calories per scoop"; or a single sign or placard listing the calorie declaration for several food items along with the names of the food items, so long as the sign or placard is located where a consumer can view the name, calorie declaration, and serving or unit of a particular item while selecting that item.
(1 ) For purposes of paragraph (b)(2)(iii)(A) of this section, "per displayed food item"; means per each discrete unit offered for sale, for example, a bagel, a slice of pizza, or a muffin.
(2 ) For purposes of paragraph (b)(2)(iii)(A) of this section, "per serving" means, for each food:
(i ) Per serving instrument used to dispense the food offered for sale, provided that the serving instrument dispenses a uniform amount of the food (e.g., a scoop or ladle);
(ii ) If a serving instrument that dispenses a uniform amount of food is not used to dispense the food, per each common household measure (e.g., cup or tablespoon) offered for sale or per unit of weight offered for sale, e.g., per quarter pound or per 4 ounces; or
(iii ) Per total number of fluid ounces in the cup in which a self-service beverage is served and, if applicable, the description of the cup size (e.g., "140 calories per 12 fluid ounces (small)").
(3 ) The calories must be declared in the following manner:
(i ) To the nearest 5-calorie increment up to and including 50 calories and to the nearest 10-calorie increment above 50 calories except that amounts less than 5 calories may be expressed as zero.
(ii ) If the calorie declaration is provided on a sign with the food's name, price, or both, the calorie declaration, accompanied by the term "Calories" or "Cal" and the amount of the serving or displayed food item on which the calories declaration is based must be in a type size no smaller than the type size of the name or price of the menu item whichever is smaller, in the same color, or a color that is at least as conspicuous as that used for that name or price, using the same contrasting background or a background at least as contrasting as that used for that name or price. If the calorie declaration is provided on a sign that does not include the food's name, price, or both, the calorie declaration, accompanied by the term "Calories" or "Cal" and the amount of the serving or displayed food item on which the calorie declaration is based must be clear and conspicuous.
(iii ) For self-service beverages, calorie declarations must be accompanied by the term "fluid ounces" and, if applicable, the description of the cup size (e.g., "small," "medium").
(B) For food that is self-service or on display and is identified by an individual sign adjacent to the food itself where such sign meets the definition of a menu or menu board under paragraph (a) of this section, the statement required by paragraph (b)(2)(i)(B) of this section and the statement required by paragraph (b)(2)(i)(C) of this section. These two statements may appear on the sign adjacent to the food itself; on a separate, larger sign, in close proximity to the food that can be easily read as the consumer is making order selections; or on a large menu board that can be easily read as the consumer is viewing the food.
(C) The nutrition information in written form required by paragraph (b)(2)(ii) of this section, except for packaged food insofar as it bears nutrition labeling information required by and in accordance with paragraph (b)(2)(ii) of this section and the packaged food, including its label, can be examined by a consumer before purchasing the food.
(c) Determination of nutrient content. (1) A covered establishment must have a reasonable basis for its nutrient declarations. Nutrient values may be determined by using nutrient databases (with or without computer software programs), cookbooks, laboratory analyses, or other reasonable means, including the use of Nutrition Facts on labels on packaged foods that comply with the nutrition labeling requirements of section 403(q)(1) of the Federal Food, Drug, and Cosmetic Act and § 101.9, FDA nutrient values for raw fruits and vegetables in Appendix C of this part, or FDA nutrient values for cooked fish in Appendix D of this part.
(2) Nutrient declarations for standard menu items must be accurate and consistent with the specific basis used to determine nutrient values. A covered establishment must take reasonable steps to ensure that the method of preparation (e.g., types and amounts of ingredients, cooking temperatures) and amount of a standard menu item offered for sale adhere to the factors on which its nutrient values were determined.
(3) A covered establishment must provide to FDA, within a reasonable period of time upon request, information substantiating nutrient values including the method and data used to derive these nutrient values. This information must include the following:
(i) For nutrient databases:
(A) The name and version (including the date of the version) of the database, and, as applicable, the name of the applicable software company and any Web site address for the database. The name and version of a database would include the name and version of the computer software, if applicable;
(B) The recipe or formula used as a basis for the nutrient declarations;
(C)(1 ) Information on:
(i ) The amount of each nutrient that the specified amount of each ingredient identified in the recipe contributes to the menu item; and
(ii ) How the database was used including calculations or operations (e.g., worksheets or computer printouts) to determine the nutrient values for the standard menu items;
(2 ) If the information in paragraph (c)(3)(i)(C)(1 ) of this section is not available, certification attesting that the database will provide accurate results when used appropriately and that the database was used in accordance with its instructions;
(D) A detailed listing (e.g., printout) of the nutrient values determined for each standard menu item.
(E) Any other information pertinent to the final nutrient values of the standard menu item (e.g., information about what might cause slight variations in the nutrient profile such as moisture variations);
(F) A statement signed and dated by a responsible individual, employed at the covered establishment or its corporate headquarters or parent entity, who can certify that the information contained in the nutrient analysis is complete and accurate; and
(G) A statement signed and dated by a responsible individual employed at the covered establishment certifying that the covered establishment has taken reasonable steps to ensure that the method of preparation (e.g., types and amounts of ingredients in the recipe, cooking temperatures) and amount of a standard menu item offered for sale adhere to the factors on which its nutrient values were determined.
(ii) For published cookbooks that contain nutritional information for recipes in the cookbook:
(A) The name, author, and publisher of the cookbook used;
(B) If available, information provided by the cookbook or from the author or publisher about how the nutrition information for the recipes was obtained;
(C) A copy of the recipe used to prepare the standard menu item and a copy of the nutrition information for that standard menu item as provided by the cookbook; and
(D) A statement signed and dated by a responsible individual employed at the covered establishment certifying that that the covered establishment has taken reasonable steps to ensure that the method of preparation (e.g., types and amounts of ingredients in the recipe, cooking temperatures) and amount of a standard menu item offered for sale adhere to the factors on which its nutrient values were determined. (Recipes may be divided as necessary to accommodate differences in the portion size derived from the recipe and that are served as the standard menu item but no changes may be made to the proportion of ingredients used.)
(iii) For laboratory analyses:
(A) A copy of the recipe for the standard menu item used for the nutrient analysis;
(B) The name and address of the laboratory performing the analysis;
(C) Copies of analytical worksheets, including the analytical method, used to determine and verify nutrition information;
(D) A statement signed and dated by a responsible individual, employed at the covered establishment or its corporate headquarters or parent entity, who can certify that the information contained in the nutrient analysis is complete and accurate; and
(E) A statement signed and dated by a responsible individual employed at the covered establishment certifying that the covered establishment has taken reasonable steps to ensure that the method of preparation (e.g., types and amounts of ingredients in the recipe, cooking temperatures) and amount of a standard menu item offered for sale adhere to the factors on which its nutrient values were determined.
(iv) For nutrition information provided by other reasonable means:
(A) A detailed description of the means used to determine the nutrition information;
(B) A recipe or formula used as a basis for the nutrient determination;
(C) Any data derived in determining the nutrient values for the standard menu item, e.g., nutrition information about the ingredients used with the source of the nutrient information;
(D) A statement signed and dated by a responsible individual, employed at the covered establishment or its corporate headquarters or parent entity, who can certify that the information contained in the nutrient analysis is complete and accurate; and
(E) A statement signed and dated by a responsible individual employed at the covered establishment certifying that the covered establishment has taken reasonable steps to ensure that the method of preparation (e.g., types and amounts of ingredients in the recipe, cooking temperatures) and amount of a standard menu item offered for sale adhere to the factors on which its nutrient values were determined.
(d) Voluntary registration to be subject to the menu labeling requirements - (1) Applicability. A restaurant or similar retail food establishment that is not part of a chain with 20 or more locations doing business under the same name and offering for sale substantially the same menu items may voluntarily register to be subject to the requirements established in this section. Restaurants and similar retail food establishments that voluntarily register will no longer be subject to non-identical State or local nutrition labeling requirements.
(2) Who may register? The authorized official of a restaurant or similar retail food establishment as defined in paragraph (a) of this section, which is not otherwise subject to paragraph (b) of this section, may register with FDA.
(3) What information is required? Authorized officials for restaurants and similar retail food establishments must provide FDA with the following information on Form FDA 3757:
(i) The contact information (including name, address, phone number, and email address) for the authorized official;
(ii) The contact information (including name, address, phone number, and email address) of each restaurant or similar retail food establishment being registered, as well as the name and contact information for an official onsite, such as the owner or manager, for each specific restaurant or similar retail food establishment;
(iii) All trade names the restaurant or similar retail food establishment uses;
(iv) Preferred mailing address (if different from location address for each establishment) for purposes of receiving correspondence; and
(v) Certification that the information submitted is true and accurate, that the person submitting it is authorized to do so, and that each registered restaurant or similar retail food establishment will be subject to the requirements of section 403(q)(5)(H) of the Federal Food, Drug, and Cosmetic Act and this section.
(4) How to register. Authorized officials of restaurants and similar retail food establishments who elect to be subject to requirements in section 403(q)(5)(H) of the Federal Food, Drug, and Cosmetic Act can register by visiting http://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm217762.htm. FDA has created a form (Form 3757) that contains fields requesting the information in paragraph (d)(3) of this section and made the form available at this Web site. Registrants must use this form to ensure that complete information is submitted.
(i) Information should be submitted by email by typing complete information into the form (PDF), saving it on the registrant's computer, and sending it by email to menulawregistration@fda.hhs.gov.
(ii) If email is not available, the registrant can either fill in the form (PDF) and print it out (or print out the blank PDF and fill in the information by hand or typewriter), and either fax the completed form to 301-436-2804 or mail it to FDA, CFSAN Menu and Vending Machine Registration, White Oak Building 22, Rm. 0209, 10903 New Hampshire Ave., Silver Spring, MD 20993.
(5) When to renew the registration. To keep the establishment's registration active, the authorized official of the restaurant or similar retail food establishment must register every other year within 60 days prior to the expiration of the establishment's current registration with FDA. Registration will automatically expire if not renewed.
(e) Signatures. Signatures obtained under paragraph (d) of this section that meet the definition of electronic signatures in § 11.3(b)(7) of this chapter are exempt from the requirements of part 11 of this chapter.
(f) Misbranding. A standard menu item offered for sale in a covered establishment shall be deemed misbranded under sections 201(n), 403(a), 403(f) and/or 403(q) of the Federal Food, Drug, and Cosmetic Act if its label or labeling is not in conformity with paragraph (b) or (c) of this section.
[79 FR 71253, Dec. 1, 2014]
|
|
|
Sec. 101.12 Reference amounts customarily consumed per eating occasion.
|
|
(a) The general principles and factors that the Food and Drug Administration (FDA) considered in arriving at the reference amounts customarily consumed per eating occasion (reference amounts) which are set forth in paragraph (b) of this section, are that:
(1) FDA calculated the reference amounts for persons 4 years of age or older to reflect the amount of food customarily consumed per eating occasion by persons in this population group. These reference amounts are based on data set forth in appropriate national food consumption surveys.
(2) FDA calculated the reference amounts for an infant or child under 4 years of age to reflect the amount of food customarily consumed per eating occasion by infants up to 12 months of age or by children 1 through 3 years of age, respectively. These reference amounts are based on data set forth in appropriate national food consumption surveys. Such reference amounts are to be used only when the food is specially formulated or processed for use by an infant or by a child under 4 years of age.
(3) An appropriate national food consumption survey includes a large sample size representative of the demographic and socioeconomic characteristics of the relevant population group and must be based on consumption data under actual conditions of use.
(4) To determine the amount of food customarily consumed per eating occasion, FDA considered the mean, median, and mode of the consumed amount per eating occasion.
(5) When survey data were insufficient, FDA took various other sources of information on serving sizes of food into consideration. These other sources of information included:
(i) Serving sizes used in dietary guidance recommendations or recommended by other authoritative systems or organizations;
(ii) Serving sizes recommended in comments;
(iii) Serving sizes used by manufacturers and grocers; and
(iv) Serving sizes used by other countries.
(6) Because they reflect the amount customarily consumed, the reference amount and, in turn, the serving size declared on the product label are based on only the edible portion of food, and not bone, seed, shell, or other inedible components.
(7) The reference amount is based on the major intended use of the food (e.g., milk as a beverage and not as an addition to cereal).
(8) The reference amounts for products that are consumed as an ingredient of other foods, but that may also be consumed in the form in which they are purchased (e.g., butter), are based on use in the form purchased.
(9) FDA sought to ensure that foods that have similar dietary usage, product characteristics, and customarily consumed amounts have a uniform reference amount.
(b) The following reference amounts shall be used as the basis for determining serving sizes for specific products:
Table 1 - Reference Amounts Customarily Consumed per Eating Occasion: Foods for Infants and Young Children 1 Through 3 Years of Age
1 2 3
| Product category
| Reference amount
| Label statement
4
|
|---|
| Cereals, dry instant | 15 g | __ cup (__ g)
| | Cereals, prepared, ready-to-serve | 110 g | __ cup(s) (__ g)
| | Other cereal and grain products, dry ready-to-eat, e.g., ready-to-eat cereals, cookies, teething biscuits, and toasts | 7 g for infants and 20 g for young children (1 through 3 years of age) for ready-to-eat cereals; 7 g for all others | __ cup(s) (__ g) for ready-to-eat cereals; piece(s) (__ g) for others
| | Dinners, deserts, fruits, vegetables or soups, dry mix | 15 g | __ tbsp(s) (__ g); __ cup(s) (__ g)
| | Dinners, desserts, fruits, vegetables or soups, ready-to-serve, junior type | 110 g | __ cup(s) (__ g); cup(s) (__ mL)
| | Dinners, desserts, fruits, vegetables or soups, ready-to-serve, strained type | 110 g | __ cup(s) (__ g); cup(s) (__ mL)
| | Dinners, stews or soups for young children, ready-to-serve | 170 g | __ cup(s) (__ g); cup(s) (__ mL)
| | Fruits for young children, ready-to-serve | 125 g | __ cup(s) (__ g)
| | Vegetables for young children, ready-to-serve | 70 g | __ cup(s) (__ g)
| | Eggs/egg yolks, ready-to serve | 55 g | __ cup(s) (__ g)
| | Juices all varieties | 120 mL | 4 fl oz (120 mL)
|
Table 2 - Reference Amounts Customarily Consumed Per Eating Occasion: General Food Supply
1 2 3
| Product category
| Reference amount
| Label statement
4
|
|---|
| Bakery Products:
| | | | Bagels, toaster pastries, muffins (excluding English muffins) | 110 g | __ piece(s) (__ g)
| | Biscuits, croissants, tortillas, soft bread sticks, soft pretzels, corn bread, hush puppies, scones, crumpets, English muffins | 55 g | __ piece(s) (__ g)
| | Breads (excluding sweet quick type), rolls | 50 g | __ piece(s) (__ g) for sliced bread and distinct pieces (e.g., rolls); 2 oz (56 g/__ inch slice) for unsliced bread
| | Bread sticks - see crackers
| | | | Toaster pastries - see bagels, toaster pastries, muffins (excluding English muffins)
| | | | Brownies | 40 g | __ piece(s) (__ g) for distinct pieces; fractional slice (__ g) for bulk
| | Cakes, heavyweight (cheese cake; pineapple upside-down cake; fruit, nut, and vegetable cakes with more than or equal to 35 percent of the finished weight as fruit, nuts, or vegetables or any of these combinations)
5 | 125 g | __ piece(s) (__ g) for distinct pieces (e.g., sliced or individually packaged products); __ fractional slice (__ g) for large discrete units
| | Cakes, mediumweight (chemically leavened cake with or without icing or filling except those classified as light weight cake; fruit, nut, and vegetable cake with less than 35 percent of the finished weight as fruit, nuts, or vegetables or any of these combinations; light weight cake with icing; Boston cream pie; cupcake; eclair; cream puff)
6 | 80 g | __ piece(s) (__ g) for distinct pieces (e.g., cupcake); __ fractional slice (__ g) for large discrete units
| | Cakes, lightweight (angel food, chiffon, or sponge cake without icing or filling)
7 | 55 g | __ piece(s) (__ g) for distinct pieces (e.g., sliced or individually packaged products); __ fractional slice (__ g) for large discrete units
| | Coffee cakes, crumb cakes, doughnuts, Danish, sweet rolls, sweet quick type breads | 55 g | __ piece(s) (__ g) for sliced bread and distinct pieces (e.g., doughnut); 2 oz (56 g/visual unit of measure) for bulk products (e.g., unsliced bread)
| | Cookies | 30 g | __ piece(s) (__ g)
| | Crackers that are usually not used as snack, melba toast, hard bread sticks, ice cream cones
8 | 15 g | __ piece(s) (__ g)
| | Crackers that are usually used as snacks | 30 g | __ piece(s) (__ g)
| | Croutons | 7 g | __ tbsp(s) (__ g); __ cup(s) (__ g); __ piece(s) (__ g) for large pieces
| | Eggroll, dumpling, wonton, or potsticker wrappers | 20 g | __ sheet (__ g); wrapper (__ g)
| | French toast, crepes, pancakes, variety mixes | 110 g prepared for French toast, crepes, and pancakes; 40 g dry mix for variety mixes | __ piece(s) (__ g); __ cup(s) (__ g) for dry mix
| | Grain-based bars with or without filling or coating, e.g., breakfast bars, granola bars, rice cereal bars | 40 g | __ piece(s) (__ g)
| | Ice cream cones - see crackers
| | | | Pies, cobblers, fruit crisps, turnovers, other pastries | 125 g | __ piece(s) (__ g) for distinct pieces; __ fractional slice (__ g) for large discrete units
| | Pie crust, pie shells, pastry sheets, (e.g., phyllo, puff pastry sheets) | the allowable declaration closest to an 8 square inch surface area | __ fractional slice(s) (__ g) for large discrete units; __ shells (__ g); __ fractional __ sheet(s) (__ g) for distinct pieces (e.g., Pastry sheet).
| | Pizza crust | 55 g | __ fractional slice (__ g)
| | Taco shells, hard | 30 g | __ shell(s) (__ g)
| | Waffles | 85 g | __ piece(s) (__ g)
| | Beverages:
| | | | Carbonated and noncarbonated beverages, wine coolers, water | 360 mL | 12 fl oz (360 mL)
| | Coffee or tea, flavored and sweetened | 360 mL prepared | 12 fl oz (360 mL)
| | Cereals and Other Grain Products:
| | | | Breakfast cereals (hot cereal type), hominy grits | 1 cup prepared; 40 g plain dry cereal; 55 g flavored, sweetened cereal | __ cup(s) (__ g)
| | Breakfast cereals, ready-to-eat, weighing less than 20 g per cup, e.g., plain puffed cereal grains | 15 g | __ cup(s) (__ g)
| | Breakfast cereals, ready-to-eat, weighing 20 g or more but less than 43 g per cup; high fiber cereals containing 28 g or more of fiber per 100 g | 40 g | __ cup(s) (__ g)
| | Breakfast cereals, ready-to-eat, weighing 43 g or more per cup; biscuit types | 60 g | __ piece(s) (__ g) for large distinct pieces (e.g., biscuit type); __ cup(s) (__ g) for all others
| | Bran or wheat germ | 15 g | __ tbsp(s) (__ g); __ cup(s) (__ g)
| | Flours or cornmeal | 30 g | __ tbsp(s) (__ g); __ cup(s) (__ g)
| | Grains, e.g., rice, barley, plain | 140 g prepared; 45 g dry | __ cup(s) (__ g)
| | Pastas, plain | 140 g prepared; 55 g dry | __ cup(s) (__ g); __ piece(s) (__ g) for large pieces (e.g., large shells or lasagna noodles) or 2 oz (56 g/visual unit of measure) for dry bulk products (e.g., spaghetti)
| | Pastas, dry, ready-to-eat, e.g., fried canned chow mein noodles | 25 g | __ cup(s) (__ g)
| | Starches, e.g., cornstarch, potato starch, tapioca, etc | 10 g | __ tbsp (__ g)
| | Stuffing | 100 g | __ cup(s) (__ g)
| | Dairy Products and Substitutes:
| | | | Cheese, cottage | 110 g | __ cup (__ g)
| | Cheese used primarily as ingredients, e.g., dry cottage cheese, ricotta cheese | 55 g | __ cup (__ g)
| | Cheese, grated hard, e.g., Parmesan, Romano | 5 g | __ tbsp (__ g)
| | Cheese, all others except those listed as separate categories - includes cream cheese and cheese spread | 30 g | __ piece(s) (__ g) for distinct pieces; __ tbsp(s) (__ g) for cream cheese and cheese spread; 1 oz (28 g/visual unit of measure) for bulk
| | Cheese sauce - see sauce category
| | | | Cream or cream substitutes, fluid | 15 mL | 1 tbsp (15 mL)
| | Cream or cream substitutes, powder | 2 g | __ tsp (__ g)
| | Cream, half & half | 30 mL | 2 tbsp (30 mL)
| | Eggnog | 120 mL |
1/2 cup (120 mL); 4 fl oz (120 mL)
| | Milk, condensed, undiluted | 30 mL | 2 tbsp (30 mL)
| | Milk, evaporated, undiluted | 30 mL | 2 tbsp (30 mL)
| | Milk, milk-substitute beverages, milk-based drinks, e.g., instant breakfast, meal replacement, cocoa, soy beverage | 240 mL | 1 cup (240 mL); 8 fl oz (240 mL)
| | Shakes or shake substitutes, e.g., dairy shake mixes, fruit frost mixes | 240 mL | 1 cup (240 mL); 8 fl oz (240 mL)
| | Sour cream | 30 g | __ tbsp (__ g)
| | Yogurt | 170 g | __ cup (__ g)
| | Desserts:
| | | | Ice cream, frozen yogurt, sherbet, frozen flavored and sweetened ice and pops, frozen fruit juices: all types bulk and novelties (e.g., bars, sandwiches, cones, cups) |
2/3 cup - includes the volume for coatings and wafers |
2/3 cup (__ g), __ piece(s) (__ g) for individually wrapped or packaged products
| | Sundae | 1 cup | 1 cup (__ g)
| | Custards, gelatin, or pudding |
1/2 cup prepared; amount to make
1/2 cup prepared when dry | __ piece(s) (__ g) for distinct unit (e.g., individually packaged products);
1/2 cup (__ g) for bulk
| | Dessert Toppings and Fillings:
| | | | Cake frostings or icings | 2 tbsp | __ tbsp(s) (__ g)
| | Other dessert toppings, e.g., fruits, syrups, spreads, marshmallow cream, nuts, dairy and non-dairy whipped toppings | 2 tbsp | 2 tbsp (__ g); 2 tbsp (30 mL)
| | Pie fillings | 85 g | __ cup(s) (__ g)
| | Egg and Egg Substitutes:
| | | | Egg mixtures, e.g., egg foo young, scrambled eggs, omelets | 110 g | __ piece(s) (__ g) for discrete pieces; __ cup(s) (__ g)
| | Eggs (all sizes)
8 | 50 g | 1 large, medium, etc. (__ g)
| | Egg whites, sugared eggs, sugared egg yolks, and egg substitutes (fresh, frozen, dried) | An amount to make 1 large (50 g) egg | __ cup(s) (__ g); __ cup(s) (__ mL)
| | Fats and Oils:
| | | | Butter, margarine, oil, shortening | 1 tbsp | 1 tbsp (__ g); 1 tbsp (15 mL)
| | Butter replacement, powder | 2 g | __ tsp(s) (__ g)
| | Dressings for salads | 30 g | __ tbsp (__ g); __ tbsp (__ mL)
| | Mayonnaise, sandwich spreads, mayonnaise-type dressings | 15 g | __ tbsp (__ g)
| | Spray types | 0.25 g | About __ seconds spray (__ g)
| | Fish, Shellfish, Game Meats,
9 and Meat or Poultry Substitutes:
| | | | Bacon substitutes, canned anchovies,
10 anchovy pastes, caviar | 15 g | __ piece(s) (__ g) for discrete pieces; __ tbsp(s) (__ g) for others
| | Dried, e.g., jerky | 30 g | __ piece(s) (__ g)
| | Entrees with sauce, e.g., fish with cream sauce, shrimp with lobster sauce | 140 g cooked | __ cup(s) (__ g); 5 oz (140 g/visual unit of measure) if not measurable by cup
| | Entrees without sauce, e.g., plain or fried fish and shellfish, fish and shellfish cake | 85 g cooked; 110 g uncooked
11 | __ piece(s) (__ g) for discrete pieces; __ cup(s) (__ g); __ oz (__ g/visual unit of measure) if not measurable by cup
12
| | Fish, shellfish, or game meat
9, canned
10 | 85 g | __ piece(s) (__ g) for discrete pieces; __ cup(s) (__ g); 3 oz (85 g/__ cup) for products that are difficult to measure the g weight of cup measure (e.g., tuna); 3 oz (85 g/__ pieces) for products that naturally vary in size (e.g., sardines)
| | Substitute for luncheon meat, meat spreads, Canadian bacon, sausages, frankfurters, and seafood | 55 g | __ piece(s) (__ g) for distinct pieces (e.g., slices, links); __ cup(s) (__ g); 2 oz (56 g/visual unit of measure) for nondiscrete bulk product
| | Smoked or pickled fish,
10 shellfish, or game meat
9; fish or shellfish spread | 55 g | __ piece(s) (__ g) for distinct pieces (e.g., slices, links) or __ cup(s) (__ g); 2 oz (56 g/visual unit of measure) for nondiscrete bulk product
| | Substitutes for bacon bits - see Miscellaneous
| | | | Fruits and Fruit Juices:
| | | | Candied or pickled
10 | 30 g | __ piece(s) (__ g)
| | Dehydrated fruits - see snack category
| | | | Dried | 40 g | __ piece(s) (__ g) for large pieces (e.g., dates, figs, prunes); __ cup(s) (__ g) for small pieces (e.g., raisins)
| | Fruits for garnish or flavor, e.g., maraschino cherries
10 | 4 g | 1 cherry (__ g); __ piece(s) (__ g)
| | Fruit relishes, e.g., cranberry sauce, cranberry relish | 70 g | __ cup(s) (__ g)
| | Fruits used primarily as ingredients, avocado | 50 g | See footnote
12
| | Fruits used primarily as ingredients, others (cranberries, lemon, lime) | 50 g | __ piece(s) (__ g) for large fruits; __ cup(s) (__ g) for small fruits measurable by cup
12
| | Watermelon | 280 g | See footnote
12
| | All other fruits (except those listed as separate categories), fresh, canned or frozen | 140 g | __ piece(s) (__ g) for large pieces (e.g., strawberries, prunes, apricots, etc.); __ cup(s) (__ g) for small pieces (e.g., blueberries, raspberries, etc.)
12
| | Juices, nectars, fruit drinks | 240 mL | 8 fl oz (240 mL)
| | Juices used as ingredients, e.g., lemon juice, lime juice | 5 mL | 1 tsp (5 mL)
| | Legumes:
| | | | Tofu,
10 tempeh | 85 g | __ piece(s) (__ g) for discrete pieces; 3 oz (84 g/visual unit of measure) for bulk products
| | Beans, plain or in sauce | 130 g for beans in sauce or canned in liquid and refried beans prepared; 90 g for others prepared; 35 g dry | __ cup (__ g)
| | Miscellaneous:
| | | | Baking powder, baking soda, pectin | 0.6 g | __ tsp (__ g)
| | Baking decorations, e.g., colored sugars and sprinkles for cookies, cake decorations | 1 tsp or 4 g if not measurable by teaspoon | __ piece(s) (__ g) for discrete pieces; 1 tsp (__ g)
| | Batter mixes, bread crumbs | 30 g | __ tbsp(s) (__ g); __ cup(s) (__ g)
| | Chewing gum
8 | 3 g | __ piece(s) (__ g)
| | Cocoa powder, carob powder, unsweetened | 1 tbsp | 1 tbsp (__ g)
| | Cooking wine | 30 mL | 2 tbsp (30 mL)
| | Dietary supplements | The maximum amount recommended, as appropriate, on the label for consumption per eating occasion or, in the absence of recommendations, 1 unit, e.g., tablet, capsule, packet, teaspoonful, etc | __ tablet(s), __ capsules(s), __ packet(s), __ tsp(s) (__ g), etc.
| | Meat, poultry, and fish coating mixes, dry; seasoning mixes, dry, e.g., chili seasoning mixes, pasta salad seasoning mixes | Amount to make one reference amount of final dish | __ tsp(s) (__ g); __ tbsp(s) (__ g)
| | Milk, milk substitute, and fruit juice concentrates (without alcohol) (e.g., drink mixers, frozen fruit juice concentrate, sweetened cocoa powder) | Amount to make 240 mL drink (without ice) | __ fl oz (__ mL); __ tsp (__ g); tbsp (__ g)
| | Drink mixes (without alcohol): All other types (e.g., flavored syrups and powdered drink mixes) | Amount to make 360 mL drink (without ice) | __ fl oz (__ mL); __ tsp (__ g); __ tbsp (__ g)
| | Salad and potato toppers, e.g., salad crunchies, salad crispins, substitutes for bacon bits | 7 g | __ tbsp(s) (__ g)
| | Salt, salt substitutes, seasoning salts (e.g., garlic salt) |
1/4 tsp |
1/4 tsp (__ g); __ piece(s) (__ g) for discrete pieces (e.g., individually packaged products)
| | Seasoning oils and seasoning sauces (e.g., coconut concentrate, sesame oil, almond oil, chili oil, coconut oil, walnut oil) | 1 tbsp | 1 tbsp (__ g)
| | Seasoning pastes (e.g., garlic paste, ginger paste, curry paste, chili paste, miso paste), fresh or frozen | 1 tsp | 1 tsp (__ g)
| | Spices, herbs (other than dietary supplements) |
1/4 tsp or 0.5 g if not measurable by teaspoon |
1/4 tsp (__ g); __ piece(s) (__ g) if not measurable by teaspoons (e.g., bay leaf)
| | Mixed Dishes:
| | | | Appetizers, hors d'oeuvres, mini mixed dishes, e.g., mini bagel pizzas, breaded mozzarella sticks, egg rolls, dumplings, potstickers, wontons, mini quesadillas, mini quiches, mini sandwiches, mini pizza rolls, potato skins | 85 g, add 35 g for products with gravy or sauce topping | __ piece(s) (__ g)
| | Measurable with cup, e.g., casseroles, hash, macaroni and cheese, pot pies, spaghetti with sauce, stews, etc | 1 cup | 1 cup (__ g)
| | Not measurable with cup, e.g., burritos, enchiladas, pizza, pizza rolls, quiche, all types of sandwiches | 140 g, add 55 g for products with gravy or sauce topping, e.g., enchilada with cheese sauce, crepe with white sauce
13 | __ piece(s) (__ g) for discrete pieces; __ fractional slice (__ g) for large discrete units
| | Nuts and Seeds:
| | | | Nuts, seeds and mixtures, all types: Sliced, chopped, slivered, and whole | 30 g | __ piece(s) (__ g) for large pieces (e.g., unshelled nuts); __ tbsp(s) (__ g); __ cup(s) (__ g) for small pieces (e.g., peanuts, sunflower seeds)
| | Nut and seed butters, pastes, or creams | 2 tbsp | 2 tbsp (__ g)
| | Coconut, nut and seed flours | 15 g | __ tbsp(s) (__ g); __ cup (__ g)
| | Potatoes and Sweet Potatoes/Yams:
| | | | French fries, hash browns, skins, or pancakes | 70 g prepared; 85 g for frozen unprepared French fries | __ piece(s) (__ g) for large distinct pieces (e.g., patties, skins); 2.5 oz (70 g/__ pieces) for prepared fries; 3 oz (84 g/__ pieces) for unprepared fries
| | Mashed, candied, stuffed or with sauce | 140 g | __ piece(s) (__ g) for discrete pieces (e.g., stuffed potato); __ cup(s) (__ g)
| | Plain, fresh, canned, or frozen | 110 g for fresh or frozen; 125 g for vacuum packed; 160 g for canned in liquid | __ piece(s) (__ g) for discrete pieces; __ cup(s) (__ g) for sliced or chopped products
| | Salads:
| | | | Gelatin salad | 120 g | __ cup (__ g)
| | Pasta or potato salad | 140 g | __ cup(s) (__ g)
| | All other salads, e.g., egg, fish, shellfish, bean, fruit, or vegetable salads | 100 g | __ cup(s) (__ g)
| | Sauces, Dips, Gravies, and Condiments:
| | | | Barbecue sauce, hollandaise sauce, tartar sauce, tomato chili sauce, other sauces for dipping (e.g., mustard sauce, sweet and sour sauce), all dips (e.g., bean dips, dairy-based dips, salsa) | 2 tbsp | 2 tbsp (__ g); 2 tbsp (30 mL)
| | Major main entree sauces, e.g., spaghetti sauce | 125 g | __ cup (__ g); __ cup (__ mL)
| | Minor main entree sauces (e.g., pizza sauce, pesto sauce, Alfredo sauce), other sauces used as toppings (e.g., gravy, white sauce, cheese sauce), cocktail sauce |
1/4 cup |
1/4 cup (__ g);
1/4 cup (60 mL)
| | Major condiments, e.g., catsup, steak sauce, soy sauce, vinegar, teriyaki sauce, marinades | 1 tbsp | 1 tbsp (__ g); 1 tbsp (15 mL)
| | Minor condiments, e.g., horseradish, hot sauces, mustards, Worcestershire sauce | 1 tsp | 1 tsp (__ g); 1 tsp (5 mL)
| | Snacks:
| | | | All varieties, chips, pretzels, popcorn, extruded snacks, fruit and vegetable-based snacks (e.g., fruit chips), grain-based snack mixes | 30 g | __ cup (__ g) for small pieces (e.g., popcorn); __ piece(s) (__ g) for large pieces (e.g., large pretzels; pressed dried fruit sheet); 1 oz (28g/visual unit of measure) for bulk products (e.g., potato chips)
| | Soups:
| | | | All varieties | 245 g | __ cup (__ g); __ cup (__ mL)
| | Dry soup mixes, bouillon | Amount to make 245 g | __ cup (__ g); __ cup (__ mL)
| | Sugars and Sweets:
| | | | Baking candies (e.g., chips) | 15 g | __ piece(s) (__ g) for large pieces; __ tbsp(s) (__ g) for small pieces;
1/2 oz (14 g/visual unit of measure) for bulk products
| | After-dinner confectioneries | 10 g | __ piece(s) (__ g)
| | Hard candies, breath mints
8 | 2 g | __ piece(s) (__ g)
| | Hard candies, roll-type, mini-size in dispenser packages | 5 g | __ piece(s) (__ g)
| | Hard candies, others; powdered candies, liquid candies | 15 mL for liquid candies; 15 g for all others | __ piece(s) (__ g) for large pieces; __ tbsp(s) (__ g) for "mini-size" candies measurable by tablespoon; __ straw(s) (__ g) for powdered candies; __ wax bottle(s) (__ mL) for liquid candies;
1/2 oz (14 g/visual unit of measure) for bulk products
| | All other candies | 30 g | __ piece(s) (__ g); 1 oz (30 g/visual unit of measure) for bulk products
| | Confectioner's sugar | 30 g | __ cup (__ g)
| | Honey, jams, jellies, fruit butter, molasses, fruit pastes, fruit chutneys | 1 tbsp | 1 tbsp (__ g); 1 tbsp (15 mL)
| | Marshmallows | 30 g | __ cup(s) (__ g) for small pieces; __ piece(s) (__ g) for large pieces
| | Sugar | 8 g | __ tsp (__ g); __ piece(s) (__ g) for discrete pieces (e.g., sugar cubes, individually packaged products)
| | Sugar substitutes | An amount equivalent to one reference amount for sugar in sweetness | __ tsp(s) (__ g) for solids; __ drop(s) (__ g) for liquid; __ piece(s) (__ g) (e.g., individually packaged products)
| | Syrups | 30 mL for all syrups | 2 tbsp (30 mL)
| | Vegetables:
| | | | Dried vegetables, dried tomatoes, sun-dried tomatoes, dried mushrooms, dried seaweed | 5 g, add 5 g for products packaged in oil | __ piece(s);
1/3 cup (__ g)
| | Dried seaweed sheets | 3 g | __ piece(s) (__ g); __ cup(s) (__ g)
| | Vegetables primarily used for garnish or flavor (e.g., pimento,
10 parsley, fresh or dried) | 4 g | __ piece(s) (__ g); __ tbsp(s) (__ g) for chopped products
| | Fresh or canned chili peppers, jalapeno peppers, other hot peppers, green onion | 30 g | __ piece(s) (__ g)
12; __ tbsp(s) (__ g); __ cup(s) (__ g) for sliced or chopped products
| | All other vegetables without sauce: Fresh, canned, or frozen | 85 g for fresh or frozen; 95 g for vacuum packed; 130 g for canned in liquid, cream-style corn, canned or stewed tomatoes, pumpkin, or winter squash | __ piece(s) (__ g) for large pieces (e.g., Brussels sprouts); __ cup(s) (__ g) for small pieces (e.g., cut corn, green peas); 3 oz (84 g/visual unit of measure) if not measurable by cup
| | All other vegetables with sauce: Fresh, canned, or frozen | 110 g | __ piece(s) (__ g) for large pieces (e.g., Brussels sprouts); __ cup(s) (__ g) for small pieces (e.g., cut corn, green peas); 4 oz (112 g/visual unit of measure) if not measurable by cup
| | Vegetable juice | 240 mL | 8 fl oz (240 mL)
| | Olives
10 | 15 g | __ piece(s) (__ g); __ tbsp(s) (__ g) for sliced products
| | Pickles and pickled vegetables, all types
10 | 30 g | 1 oz (28 g/visual unit of measure)
| | Pickle relishes | 15 g | __ tbsp (__ g)
| | Sprouts, all types: Fresh or canned | 1/4 cup |
1/4 cup (__ g)
| | Vegetable pastes, e.g., tomato paste | 30 g | __ tbsp (__ g)
| | Vegetable sauces or purees, e.g., tomato sauce, tomato puree | 60 g | __ cup (__ g); __ cup (__ mL)
|
(c) If a product requires further preparation, e.g., cooking or the addition of water or other ingredients, and if paragraph (b) of this section provides a reference amount for the product in the prepared form, but not the unprepared form, then the reference amount for the unprepared product must be the amount of the unprepared product required to make the reference amount for the prepared product as established in paragraph (b) of this section.
(d) The reference amount for an imitation or substitute food or altered food, such as a "low calorie" version, shall be the same as for the food for which it is offered as a substitute.
(e) If a food is modified by incorporating air (aerated), and thereby the density of the food is lowered by 25 percent or more in weight than that of an appropriate reference regular food as described in § 101.13(j)(1)(ii)(A), and the reference amount of the regular food is in grams, the manufacturer may determine the reference amount of the aerated food by adjusting for the difference in density of the aerated food relative to the density of the appropriate reference food provided that the manufacturer will show FDA detailed protocol and records of all data that were used to determine the density-adjusted reference amount for the aerated food. The reference amount for the aerated food shall be rounded to the nearest 5-g increment. Such products shall bear a descriptive term indicating that extra air has been incorporated (e.g., whipped, aerated). The density-adjusted reference amounts described in paragraph (b) of this section may not be used for cakes except for cheese cake. The differences in the densities of different types of cakes having different degrees of air incorporation have already been taken into consideration in determining the reference amounts for cakes in § 101.12(b). In determining the difference in density of the aerated and the regular food, the manufacturer shall adhere to the following:
(1) The regular and the aerated product must be the same in size, shape, and volume. To compare the densities of products having nonsmooth surfaces (e.g., waffles), manufacturers shall use a device or method that ensures that the volumes of the regular and the aerated products are the same.
(2) Sample selections for the density measurements shall be done in accordance with the provisions in § 101.9(g).
(3) Density measurements of the regular and the aerated products shall be conducted by the same trained operator using the same methodology (e.g., the same equipment, procedures, and techniques) under the same conditions.
(4) Density measurements shall be replicated a sufficient number of times to ensure that the average of the measurements is representative of the true differences in the densities of the regular and the "aerated" products.
(f) For products that have no reference amount listed in paragraph (b) of this section for the unprepared or the prepared form of the product and that consist of two or more foods packaged and presented to be consumed together (e.g., peanut butter and jelly, cracker and cheese pack, pancakes and syrup, cake and frosting), the reference amount for the combined product shall be determined using the following rules:
(1) The reference amount for the combined product must be the reference amount, as established in paragraph (b) of this section, for the ingredient that is represented as the main ingredient (e.g., peanut butter, pancakes, cake) plus proportioned amounts of all minor ingredients.
(2) If the reference amounts are in compatible units, the weights or volumes must be summed (e.g., the reference amount for equal volumes of peanut butter and jelly for which peanut butter is represented as the main ingredient would be 4 tablespoons (tbsp) (2 tbsp peanut butter plus 2 tbsp jelly)). If the reference amounts are in incompatible units, all amounts must be converted to weights and summed, e.g., the reference amount for pancakes and syrup would be 110 g (the reference amount for pancakes) plus the weight of the proportioned amount of syrup.
(g) The reference amounts set forth in paragraphs (b) through (f) of this section shall be used in determining whether a product meets the criteria for nutrient content claims, such as "low calorie," and for health claims. If the serving size declared on the product label differs from the reference amount, and the product meets the criteria for the claim only on the basis of the reference amount, the claim shall be followed by a statement that sets forth the basis on which the claim is made. That statement shall include the reference amount as it appears in paragraph (b) of this section followed, in parenthesis, by the amount in common household measure if the reference amount is expressed in measures other than common household measures (e.g., for a beverage, "Very low sodium, 35 mg or less per 240 mL (8 fl oz)").
(h) The Commissioner of Food and Drugs, either on his or her own initiative or in response to a petition submitted pursuant to part 10 of this chapter, may issue a proposal to establish or amend a reference amount in paragraph (b) of this section. A petition to establish or amend a reference amount shall include:
(1) Objective of the petition;
(2) A description of the product;
(3) A complete sample product label including nutrition label, using the format established by regulation;
(4) A description of the form (e.g., dry mix, frozen dough) in which the product will be marketed;
(5) The intended dietary uses of the product with the major use identified (e.g., milk as a beverage and chips as a snack);
(6) If the intended use is primarily as an ingredient in other foods, list of foods or food categories in which the product will be used as an ingredient with information on the prioritization of the use;
(7) The population group for which the product will be offered for use (e.g., infants, children under 4 years of age);
(8) The names of the most closely related products (or in the case of foods for special dietary use and imitation or substitute foods, the names of the products for which they are offered as substitutes);
(9) The suggested reference amount (the amount of edible portion of food as consumed, excluding bone, seed, shell, or other inedible components) for the population group for which the product is intended with full description of the methodology and procedures that were used to determine the suggested reference amount. In determining the reference amount, general principles and factors in paragraph (a) of this section should be followed.
(10) The suggested reference amount shall be expressed in metric units. Reference amounts for fluids shall be expressed in milliliters. Reference amounts for other foods shall be expressed in grams except when common household units such as cups, tablespoons, and teaspoons, are more appropriate or are more likely to promote uniformity in serving sizes declared on product labels. For example, common household measures would be more appropriate if products within the same category differ substantially in density, such as frozen desserts.
(i) In expressing the reference amounts in milliliters, the following rules shall be followed:
(A) For volumes greater than 30 milliliters (mL), the volume shall be expressed in multiples of 30 mL.
(B) For volumes less than 30 mL, the volume shall be expressed in milliliters equivalent to a whole number of teaspoons or 1 tbsp, i.e., 5, 10, or 15 mL.
(ii) In expressing the reference amounts in grams, the following general rules shall be followed:
(A) For quantities greater than 10 g, the quantity shall be expressed in the nearest 5-g increment.
(B) For quantities less than 10 g, exact gram weights shall be used.
(11) A petition to create a new subcategory of food with its own reference amount shall include the following additional information:
(i) Data that demonstrate that the new subcategory of food will be consumed in amounts that differ enough from the reference amount for the parent category to warrant a separate reference amount. Data must include sample size; and the mean, standard deviation, median, and modal consumed amount per eating occasion for the petitioned product and for other products in the category, excluding the petitioned product. All data must be derived from the same survey data.
(ii) Documentation supporting the difference in dietary usage and product characteristics that affect the consumption size that distinguishes the petitioned product from the rest of the products in the category.
(12) A claim for categorical exclusion under § 25.30 or § 25.32 of this chapter or an environmental assessment under § 25.40 of this chapter, and
(13) In conducting research to collect or process food consumption data in support of the petition, the following general guidelines should be followed.
(i) Sampled population selected should be representative of the demographic and socioeconomic characteristics of the target population group for which the food is intended.
(ii) Sample size (i.e., number of eaters) should be large enough to give reliable estimates for customarily consumed amounts.
(iii) The study protocol should identify potential biases and describe how potential biases are controlled for or, if not possible to control, how they affect interpretation of results.
(iv) The methodology used to collect or process data should be fully documented and should include: study design, sampling procedures, materials used (e.g., questionnaire, and interviewer's manual), procedures used to collect or process data, methods or procedures used to control for unbiased estimates, and procedures used to correct for nonresponse.
(14) A statement concerning the feasibility of convening associations, corporations, consumers, and other interested parties to engage in negotiated rulemaking to develop a proposed rule consistent with the Negotiated Rulemaking Act (5 U.S.C. 561).
[58 FR 44051, Aug. 18, 1993; 58 FR 60109, Nov. 15, 1993, as amended at 59 FR 371, Jan. 4, 1994; 59 FR 24039, May 10, 1994; 62 FR 40598, July 29, 1997; 62 FR 49848, Sept. 23, 1997; 63 FR 14818, Mar. 27, 1998; 64 FR 12890, Mar. 16, 1999; 66 FR 56035, Nov. 6, 2001; 81 FR 34041, May 27, 2016]
|
|
|
Sec. 101.13 Nutrient content claims - general principles.
|
|
(a) This section and the regulations in subpart D of this part apply to foods that are intended for human consumption and that are offered for sale, including conventional foods and dietary supplements.
(b) A claim that expressly or implicitly characterizes the level of a nutrient of the type required to be in nutrition labeling under § 101.9 or under § 101.36 (that is, a nutrient content claim) may not be made on the label or in labeling of foods unless the claim is made in accordance with this regulation and with the applicable regulations in subpart D of this part or in part 105 or part 107 of this chapter.
(1) An expressed nutrient content claim is any direct statement about the level (or range) of a nutrient in the food, e.g., "low sodium" or "contains 100 calories."
(2) An implied nutrient content claim is any claim that:
(i) Describes the food or an ingredient therein in a manner that suggests that a nutrient is absent or present in a certain amount (e.g., "high in oat bran"); or
(ii) Suggests that the food, because of its nutrient content, may be useful in maintaining healthy dietary practices and is made in association with an explicit claim or statement about a nutrient (e.g., "healthy, contains 3 grams (g) of fat").
(3) Except for claims regarding vitamins and minerals described in paragraph (q)(3) of this section, no nutrient content claims may be made on food intended specifically for use by infants and children less than 2 years of age unless the claim is specifically provided for in parts 101, 105, or 107 of this chapter.
(4) Reasonable variations in the spelling of the terms defined in part 101 and their synonyms are permitted provided these variations are not misleading (e.g., "hi" or "lo").
(5) For dietary supplements, claims for calories, fat, saturated fat, and cholesterol may not be made on products that meet the criteria in § 101.60(b)(1) or (b)(2) for "calorie free" or "low calorie" claims, except, in the case of calorie claims, when an equivalent amount of a similar dietary supplement (e.g., another protein supplement) that the labeled food resembles and for which it substitutes, normally exceeds the definition for "low calorie" in § 101.60(b)(2).
(c) Information that is required or permitted by § 101.9 or § 101.36, as applicable, to be declared in nutrition labeling, and that appears as part of the nutrition label, is not a nutrient content claim and is not subject to the requirements of this section. If such information is declared elsewhere on the label or in labeling, it is a nutrient content claim and is subject to the requirements for nutrient content claims.
(d) A "substitute" food is one that may be used interchangeably with another food that it resembles, i.e., that it is organoleptically, physically, and functionally (including shelf life) similar to, and that it is not nutritionally inferior to unless it is labeled as an "imitation."
(1) If there is a difference in performance characteristics that materially limits the use of the food, the food may still be considered a substitute if the label includes a disclaimer adjacent to the most prominent claim as defined in paragraph (j)(2)(iii) of this section, informing the consumer of such difference (e.g., "not recommended for frying").
(2) This disclaimer shall be in easily legible print or type and in a size no less than that required by § 101.7(i) for the net quantity of contents statement, except where the size of the claim is less than two times the required size of the net quantity of contents statement, in which case the disclaimer shall be no less than one-half the size of the claim but no smaller than one-sixteenth of an inch, unless the package complies with § 101.2(c)(5), in which case the disclaimer may be in type of not less than one thirty-second of an inch.
(e)(1) Because the use of a "free" or "low" claim before the name of a food implies that the food differs from other foods of the same type by virtue of its having a lower amount of the nutrient, only foods that have been specially processed, altered, formulated, or reformulated so as to lower the amount of the nutrient in the food, remove the nutrient from the food, or not include the nutrient in the food, may bear such a claim (e.g., "low sodium potato chips").
(2) Any claim for the absence of a nutrient in a food, or that a food is low in a nutrient when the food has not been specially processed, altered, formulated, or reformulated to qualify for that claim shall indicate that the food inherently meets the criteria and shall clearly refer to all foods of that type and not merely to the particular brand to which the labeling attaches (e.g., "corn oil, a sodium-free food").
(f) A nutrient content claim shall be in type size no larger than two times the statement of identity and shall not be unduly prominent in type style compared to the statement of identity.
(g) [Reserved]
(h)(1) If a food, except a meal product as defined in § 101.13(l), a main dish product as defined in § 101.13(m), or food intended specifically for use by infants and children less than 2 years of age, contains more than 13.0 g of fat, 4.0 g of saturated fat, 60 milligrams (mg) of cholesterol, or 480 mg of sodium per reference amount customarily consumed, per labeled serving, or, for a food with a reference amount customarily consumed of 30 g or less or 2 tablespoons or less, per 50 g (for dehydrated foods that must be reconstituted before typical consumption with water or a diluent containing an insignificant amount, as defined in § 101.9(f)(1), of all nutrients per reference amount customarily consumed, the per 50 g criterion refers to the "as prepared" form), then that food must bear a statement disclosing that the nutrient exceeding the specified level is present in the food as follows: "See nutrition information for ____ content" with the blank filled in with the identity of the nutrient exceeding the specified level, e.g., "See nutrition information for fat content."
(2) If a food is a meal product as defined in § 101.13(l), and contains more than 26 g of fat, 8.0 g of saturated fat, 120 mg of cholesterol, or 960 mg of sodium per labeled serving, then that food must disclose, in accordance with the requirements as provided in paragraph (h)(1) of this section, that the nutrient exceeding the specified level is present in the food.
(3) If a food is a main dish product as defined in § 101.13(m), and contains more than 19.5 g of fat, 6.0 g of saturated fat, 90 mg of cholesterol, or 720 mg of sodium per labeled serving, then that food must disclose, in accordance with the requirements as provided in paragraph (h)(1) of this section, that the nutrient exceeding the specified level is present in the food.
(4)(i) The disclosure statement "See nutrition information for ____ content" shall be in easily legible boldface print or type, in distinct contrast to other printed or graphic matter, and in a size no less than that required by § 101.7(i) for the net quantity of contents statement, except where the size of the claim is less than two times the required size of the net quantity of contents statement, in which case the disclosure statement shall be no less than one-half the size of the claim but no smaller than one-sixteenth of an inch, unless the package complies with § 101.2(c)(2), in which case the disclosure statement may be in type of not less than one thirty-second of an inch.
(ii) The disclosure statement shall be immediately adjacent to the nutrient content claim and may have no intervening material other than, if applicable, other information in the statement of identity or any other information that is required to be presented with the claim under this section (e.g., see paragraph (j)(2) of this section) or under a regulation in subpart D of this part (e.g., see §§ 101.54 and 101.62). If the nutrient content claim appears on more than one panel of the label, the disclosure statement shall be adjacent to the claim on each panel except for the panel that bears the nutrition information where it may be omitted.
(iii) If a single panel of a food label or labeling contains multiple nutrient content claims or a single claim repeated several times, a single disclosure statement may be made. The statement shall be adjacent to the claim that is printed in the largest type on that panel.
(i) Except as provided in § 101.9 or § 101.36, as applicable, or in paragraph (q)(3) of this section, the label or labeling of a product may contain a statement about the amount or percentage of a nutrient if:
(1) The use of the statement on the food implicitly characterizes the level of the nutrient in the food and is consistent with a definition for a claim, as provided in subpart D of this part, for the nutrient that the label addresses. Such a claim might be, "less than 3 g of fat per serving;"
(2) The use of the statement on the food implicitly characterizes the level of the nutrient in the food and is not consistent with such a definition, but the label carries a disclaimer adjacent to the statement that the food is not "low" in or a "good source" of the nutrient, such as "only 200 mg sodium per serving, not a low sodium food." The disclaimer must be in easily legible print or type and in a size no less than that required by § 101.7(i) for the net quantity of contents statement except where the size of the claim is less than two times the required size of the net quantity of contents statement, in which case the disclaimer shall be no less than one-half the size of the claim but no smaller than one-sixteenth of an inch unless the package complies with § 101.2(c)(5), in which case the disclaimer may be in type of not less than one thirty-second of an inch, or
(3) The statement does not in any way implicitly characterize the level of the nutrient in the food and it is not false or misleading in any respect (e.g., "100 calories" or "5 grams of fat"), in which case no disclaimer is required.
(4) "Percent fat free" claims are not authorized by this paragraph. Such claims shall comply with § 101.62(b)(6).
(j) A food may bear a statement that compares the level of a nutrient in the food with the level of a nutrient in a reference food. These statements shall be known as "relative claims" and include "light," "reduced," "less" (or "fewer"), and "more" claims.
(1) To bear a relative claim about the level of a nutrient, the amount of that nutrient in the food must be compared to an amount of nutrient in an appropriate reference food as specified below.
(i)(A) For "less" (or "fewer") and "more" claims, the reference food may be a dissimilar food within a product category that can generally be substituted for one another in the diet (e.g., potato chips as reference for pretzels, orange juice as a reference for vitamin C tablets) or a similar food (e.g., potato chips as reference for potato chips, one brand of multivitamin as reference for another brand of multivitamin).
(B) For "light," "reduced," "added," "extra," "plus," "fortified," and "enriched" claims, the reference food shall be a similar food (e.g., potato chips as a reference for potato chips, one brand of multivitamin for another brand of multivitamin), and
(ii)(A) For "light" claims, the reference food shall be representative of the type of food that includes the product that bears the claim. The nutrient value for the reference food shall be representative of a broad base of foods of that type; e.g., a value in a representative, valid data base; an average value determined from the top three national (or regional) brands, a market basket norm; or, where its nutrient value is representative of the food type, a market leader. Firms using such a reference nutrient value as a basis for a claim, are required to provide specific information upon which the nutrient value was derived, on request, to consumers and appropriate regulatory officials.
(B) For relative claims other than "light," including "less" and "more" claims, the reference food may be the same as that provided for "light" in paragraph (j)(1)(ii)(A) of this section, or it may be the manufacturer's regular product, or that of another manufacturer, that has been offered for sale to the public on a regular basis for a substantial period of time in the same geographic area by the same business entity or by one entitled to use its trade name. The nutrient values used to determine the claim when comparing a single manufacturer's product to the labeled product shall be either the values declared in nutrition labeling or the actual nutrient values, provided that the resulting label is internally consistent to (i.e., that the values stated in the nutrition information, the nutrient values in the accompanying information and the declaration of the percentage of nutrient by which the food has been modified are consistent and will not cause consumer confusion when compared), and that the actual modification is at least equal to the percentage specified in the definition of the claim.
(2) For foods bearing relative claims:
(i) The label or labeling must state the identity of the reference food and the percentage (or fraction) of the amount of the nutrient in the reference food by which the nutrient in the labeled food differs (e.g., "50 percent less fat than (reference food)" or "1/3 fewer calories than (reference food)"),
(ii) This information shall be immediately adjacent to the most prominent claim. The type size shall be in accordance with paragraph (h)(4)(i) of this section.
(iii) The determination of which use of the claim is in the most prominent location on the label or labeling will be made based on the following factors, considered in order:
(A) A claim on the principal display panel adjacent to the statement of identity;
(B) A claim elsewhere on the principal display panel;
(C) A claim on the information panel; or
(D) A claim elsewhere on the label or labeling.
(iv) The label or labeling must also bear:
(A) Clear and concise quantitative information comparing the amount of the subject nutrient in the product per labeled serving with that in the reference food; and
(B) This statement shall appear adjacent to the most prominent claim or to the nutrition label, except that if the nutrition label is on the information panel, the quantitative information may be located elsewhere on the information panel in accordance with § 101.2.
(3) A relative claim for decreased levels of a nutrient may not be made on the label or in labeling of a food if the nutrient content of the reference food meets the requirement for a "low" claim for that nutrient (e.g., 3 g fat or less).
(k) The term "modified" may be used in the statement of identity of a food that bears a relative claim that complies with the requirements of this part, followed immediately by the name of the nutrient whose content has been altered (e.g., "Modified fat cheesecake"). This statement of identity must be immediately followed by the comparative statement such as "Contains 35 percent less fat than ______." The label or labeling must also bear the information required by paragraph (j)(2) of this section in the manner prescribed.
(l) For purposes of making a claim, a "meal product shall be defined as a food that:
(1) Makes a major contribution to the total diet by:
(i) Weighing at least 10 ounces (oz) per labeled serving; and
(ii) Containing not less than three 40-g portions of food, or combinations of foods, from two or more of the following four food groups, except as noted in paragraph (l)(1)(ii)(E) of this section.
(A) Bread, cereal, rice, and pasta group;
(B) Fruits and vegetables group;
(C) Milk, yogurt, and cheese group;
(D) Meat, poultry, fish, dry beans, eggs, and nuts group; except that;
(E) These foods shall not be sauces (except for foods in the above four food groups that are in the sauces), gravies, condiments, relishes, pickles, olives, jams, jellies, syrups, breadings or garnishes; and
(2) Is represented as, or is in a form commonly understood to be, a breakfast, lunch, dinner, or meal. Such representations may be made either by statements, photographs, or vignettes.
(m) For purposes of making a claim, a "main dish product" shall be defined as a food that:
(1) Makes a major contribution to a meal by
(i) Weighing at least 6 oz per labeled serving; and
(ii) Containing not less than 40 g of food, or combinations of foods, from each of at least two of the following four food groups, except as noted in paragraph (m)(1)(ii)(E) of this section.
(A) Bread, cereal, rice, and pasta group;
(B) Fruits and vegetables group;
(C) Milk, yogurt, and cheese group;
(D) Meat, poultry, fish, dry beans, eggs, and nuts groups; except that:
(E) These foods shall not be sauces (except for foods in the above four food groups that are in the sauces) gravies, condiments, relishes, pickles, olives, jams, jellies, syrups, breadings, or garnishes; and
(2) Is represented as, or is in a form commonly understood to be, a main dish (e.g., not a beverage or a dessert). Such representations may be made either by statements, photographs, or vignettes.
(n) Nutrition labeling in accordance with § 101.9, § 101.10, or § 101.36, as applicable, shall be provided for any food for which a nutrient content claim is made.
(o) Except as provided in § 101.10, compliance with requirements for nutrient content claims in this section and in the regulations in subpart D of this part, will be determined using the analytical methodology prescribed for determining compliance with nutrition labeling in § 101.9.
(p)(1) Unless otherwise specified, the reference amount customarily consumed set forth in § 101.12(b) through (f) shall be used in determining whether a product meets the criteria for a nutrient content claim. If the serving size declared on the product label differs from the reference amount customarily consumed, and the amount of the nutrient contained in the labeled serving does not meet the maximum or minimum amount criterion in the definition for the descriptor for that nutrient, the claim shall be followed by the criteria for the claim as required by § 101.12(g) (e.g., "very low sodium, 35 mg or less per 240 milliliters (8 fl oz.)").
(2) The criteria for the claim shall be immediately adjacent to the most prominent claim in easily legible print or type and in a size in accordance with paragraph (h)(4)(i) of this section.
(q) The following exemptions apply:
(1) Nutrient content claims that have not been defined by regulation and that are contained in the brand name of a specific food product that was the brand name in use on such food before October 25, 1989, may continue to be used as part of that brand name for such product, provided that they are not false or misleading under section 403(a) of the Federal Food, Drug, and Cosmetic Act (the act). However, foods bearing such claims must comply with section 403(f), (g), and (h) of the act;
(2) A soft drink that used the term diet as part of its brand name before October 25, 1989, and whose use of that term was in compliance with § 105.66 of this chapter as that regulation appeared in the Code of Federal Regulations on that date, may continue to use that term as part of its brand name, provided that its use of the term is not false or misleading under section 403(a) of the act. Such claims are exempt from the requirements of section 403(r)(2) of the act (e.g., the disclosure statement also required by § 101.13(h)). Soft drinks marketed after October 25, 1989, may use the term "diet" provided they are in compliance with the current § 105.66 of this chapter and the requirements of § 101.13.
(3)(i) A statement that describes the percentage of a vitamin or mineral in the food, including foods intended specifically for use by infants and children less than 2 years of age, in relation to a Reference Daily Intake (RDI) as defined in § 101.9 may be made on the label or in labeling of a food without a regulation authorizing such a claim for a specific vitamin or mineral unless such claim is expressly prohibited by regulation under section 403(r)(2)(A)(vi) of the act.
(ii) Percentage claims for dietary supplements. Under section 403(r)(2)(F) of the act, a statement that characterizes the percentage level of a dietary ingredient for which a reference daily intake (RDI) or daily reference value (DRV) has not been established may be made on the label or in labeling of dietary supplements without a regulation that specifically defines such a statement. All such claims shall be accompanied by any disclosure statement required under paragraph (h) of this section.
(A) Simple percentage claims. Whenever a statement is made that characterizes the percentage level of a dietary ingredient for which there is no RDI or DRV, the statement of the actual amount of the dietary ingredient per serving shall be declared next to the percentage statement (e.g., "40 percent omega-3 fatty acids, 10 mg per capsule").
(B) Comparative percentage claims. Whenever a statement is made that characterizes the percentage level of a dietary ingredient for which there is no RDI or DRV and the statement draws a comparison to the amount of the dietary ingredient in a reference food, the reference food shall be clearly identified, the amount of that food shall be identified, and the information on the actual amount of the dietary ingredient in both foods shall be declared in accordance with paragraph (j)(2)(iv) of this section (e.g., "twice the omega-3 fatty acids per capsule (80 mg) as in 100 mg of menhaden oil (40 mg)").
(4) The requirements of this section do not apply to:
(i) Infant formulas subject to section 412(h) of the act; and
(ii) Medical foods defined by section 5(b) of the Orphan Drug Act.
(5) A nutrient content claim used on food that is served in restaurants or other establishments in which food is served for immediate human consumption or which is sold for sale or use in such establishments shall comply with the requirements of this section and the appropriate definition in subpart D of this part, except that:
(i) Such claim is exempt from the requirements for disclosure statements in paragraph (h) of this section and §§ 101.54(d), 101.62(c), (d)(1)(ii)(D), (d)(2)(iii)(C), (d)(3), (d)(4)(ii)(C), and (d)(5)(ii)(C); and
(ii) In lieu of analytical testing, compliance may be determined using a reasonable basis for concluding that the food that bears the claim meets the definition for the claim. This reasonable basis may derive from recognized data bases for raw and processed foods, recipes, and other means to compute nutrient levels in the foods or meals and may be used provided reasonable steps are taken to ensure that the method of preparation adheres to the factors on which the reasonable basis was determined (e.g., types and amounts of ingredients, cooking temperatures, etc.). Firms making claims on foods based on this reasonable basis criterion are required to provide to appropriate regulatory officials on request the specific information on which their determination is based and reasonable assurance of operational adherence to the preparation methods or other basis for the claim; and
(iii) A term or symbol that may in some contexts constitute a claim under this section may be used, provided that the use of the term or symbol does not characterize the level of a nutrient, and a statement that clearly explains the basis for the use of the term or symbol is prominently displayed and does not characterize the level of a nutrient. For example, a term such as "lite fare" followed by an asterisk referring to a note that makes clear that in this restaurant "lite fare" means smaller portion sizes than normal; or an item bearing a symbol referring to a note that makes clear that this item meets the criteria for the dietary guidance established by a recognized dietary authority would not be considered a nutrient content claim under § 101.13.
(6) Nutrient content claims that were part of the common or usual names of foods that were subject to a standard of identity on November 8, 1990, are not subject to the requirements of paragraphs (b) and (h) of this section or to definitions in subpart D of this part.
(7) Implied nutrient content claims may be used as part of a brand name, provided that the use of the claim has been authorized by the Food and Drug Administration. Petitions requesting approval of such a claim may be submitted under § 101.69(o).
(8) The term fluoridated, fluoride added or with added fluoride may be used on the label or in labeling of bottled water that contains added fluoride.
[58 FR 2410, Jan. 6, 1993; 58 FR 17341, 17342, Apr. 2, 1993, as amended at 58 FR 44030, Aug. 18, 1993; 59 FR 393, Jan. 4, 1994; 59 FR 15051, Mar. 31, 1994; 60 FR 17205, Apr. 5, 1995; 61 FR 11731, Mar. 22, 1996; 61 FR 40332, Aug. 2, 1996; 61 FR 67452, Dec. 23, 1996; 62 FR 31339, June 9, 1997; 62 FR 49867, Sept. 23, 1997; 63 FR 14818, Mar. 27, 1998; 63 FR 26980, May 15, 1998; 81 FR 59131, Aug. 29, 2016]
|
|
|
Sec. 101.14 Health claims: general requirements.
|
|
(a) Definitions. For purposes of this section, the following definitions apply:
(1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes, characterizes the relationship of any substance to a disease or health-related condition. Implied health claims include those statements, symbols, vignettes, or other forms of communication that suggest, within the context in which they are presented, that a relationship exists between the presence or level of a substance in the food and a disease or health-related condition.
(2) Substance means a specific food or component of food, regardless of whether the food is in conventional food form or a dietary supplement that includes vitamins, minerals, herbs, or other similar nutritional substances.
(3) Nutritive value means a value in sustaining human existence by such processes as promoting growth, replacing loss of essential nutrients, or providing energy.
(4) Disqualifying nutrient levels means the levels of total fat, saturated fat, cholesterol, or sodium in a food above which the food will be disqualified from making a health claim. These levels are 13.0 grams (g) of fat, 4.0 g of saturated fat, 60 milligrams (mg) of cholesterol, or 480 mg of sodium, per reference amount customarily consumed, per label serving size, and, only for foods with reference amounts customarily consumed of 30 g or less or 2 tablespoons or less, per 50 g. For dehydrated foods that must have water added to them prior to typical consumption, the per 50-g criterion refers to the as prepared form. Any one of the levels, on a per reference amount customarily consumed, a per label serving size or, when applicable, a per 50 g basis, will disqualify a food from making a health claim unless an exception is provided in subpart E of this part, except that:
(i) The levels for a meal product as defined in § 101.13(l) are 26.0 g of fat, 8.0 g of saturated fat, 120 mg of cholesterol, or 960 mg of sodium per label serving size, and
(ii) The levels for a main dish product as defined in § 101.13(m) are 19.5 g of fat, 6.0 g of saturated fat, 90 mg of cholesterol, or 720 mg of sodium per label serving size.
(5) Disease or health-related condition means damage to an organ, part, structure, or system of the body such that it does not function properly (e.g., cardiovascular disease), or a state of health leading to such dysfunctioning (e.g., hypertension); except that diseases resulting from essential nutrient deficiencies (e.g., scurvy, pellagra) are not included in this definition (claims pertaining to such diseases are thereby not subject to § 101.14 or § 101.70).
(b) Eligibility. For a substance to be eligible for a health claim:
(1) The substance must be associated with a disease or health-related condition for which the general U.S. population, or an identified U.S. population subgroup (e.g., the elderly) is at risk, or, alternatively, the petition submitted by the proponent of the claim otherwise explains the prevalence of the disease or health-related condition in the U.S. population and the relevance of the claim in the context of the total daily diet and satisfies the other requirements of this section.
(2) If the substance is to be consumed as a component of a conventional food at decreased dietary levels, the substance must be a nutrient listed in 21 U.S.C. 343(q)(1)(C) or (q)(1)(D), or one that the Food and Drug Administration (FDA) has required to be included in the label or labeling under 21 U.S.C. 343(q)(2)(A); or
(3) If the substance is to be consumed at other than decreased dietary levels:
(i) The substance must, regardless of whether the food is a conventional food or a dietary supplement, contribute taste, aroma, or nutritive value, or any other technical effect listed in § 170.3(o) of this chapter, to the food and must retain that attribute when consumed at levels that are necessary to justify a claim; and
(ii) The substance must be a food or a food ingredient or a component of a food ingredient whose use at the levels necessary to justify a claim has been demonstrated by the proponent of the claim, to FDA's satisfaction, to be safe and lawful under the applicable food safety provisions of the Federal Food, Drug, and Cosmetic Act.
(c) Validity requirement. FDA will promulgate regulations authorizing a health claim only when it determines, based on the totality of publicly available scientific evidence (including evidence from well-designed studies conducted in a manner which is consistent with generally recognized scientific procedures and principles), that there is significant scientific agreement, among experts qualified by scientific training and experience to evaluate such claims, that the claim is supported by such evidence.
(d) General health claim labeling requirements. (1) When FDA determines that a health claim meets the validity requirements of paragraph (c) of this section, FDA will propose a regulation in subpart E of this part to authorize the use of that claim. If the claim pertains to a substance not provided for in § 101.9 or § 101.36, FDA will propose amending that regulation to include declaration of the substance.
(2) When FDA has adopted a regulation in subpart E of this part providing for a health claim, firms may make claims based on the regulation in subpart E of this part, provided that:
(i) All label or labeling statements about the substance-disease relationship that is the subject of the claim are based on, and consistent with, the conclusions set forth in the regulations in subpart E of this part;
(ii) The claim is limited to describing the value that ingestion (or reduced ingestion) of the substance, as part of a total dietary pattern, may have on a particular disease or health-related condition;
(iii) The claim is complete, truthful, and not misleading. Where factors other than dietary intake of the substance affect the relationship between the substance and the disease or health-related condition, such factors may be required to be addressed in the claim by a specific regulation in subpart E of this part;
(iv) All information required to be included in the claim appears in one place without other intervening material, except that the principal display panel of the label or labeling may bear the reference statement, "See ______ for information about the relationship between ______ and ______," with the blanks filled in with the location of the labeling containing the health claim, the name of the substance, and the disease or health-related condition (e.g., "See attached pamphlet for information about calcium and osteoporosis"), with the entire claim appearing elsewhere on the other labeling, Provided that, where any graphic material (e.g., a heart symbol) constituting an explicit or implied health claim appears on the label or labeling, the reference statement or the complete claim shall appear in immediate proximity to such graphic material;
(v) The claim enables the public to comprehend the information provided and to understand the relative significance of such information in the context of a total daily diet; and
(vi) If the claim is about the effects of consuming the substance at decreased dietary levels, the level of the substance in the food is sufficiently low to justify the claim. To meet this requirement, if a definition for use of the term low has been established for that substance under this part, the substance must be present at a level that meets the requirements for use of that term, unless a specific alternative level has been established for the substance in subpart E of this part. If no definition for "low" has been established, the level of the substance must meet the level established in the regulation authorizing the claim; or
(vii) If the claim is about the effects of consuming the substance at other than decreased dietary levels, the level of the substance is sufficiently high and in an appropriate form to justify the claim. To meet this requirement, if a definition for use of the term high for that substance has been established under this part, the substance must be present at a level that meets the requirements for use of that term, unless a specific alternative level has been established for the substance in subpart E of this part. If no definition for "high" has been established (e.g., where the claim pertains to a food either as a whole food or as an ingredient in another food), the claim must specify the daily dietary intake necessary to achieve the claimed effect, as established in the regulation authorizing the claim; Provided That:
(A) Where the food that bears the claim meets the requirements of paragraphs (d)(2)(vi) or (d)(2)(vii) of this section based on its reference amount customarily consumed, and the labeled serving size differs from that amount, the claim shall be followed by a statement explaining that the claim is based on the reference amount rather than the labeled serving size (e.g., "Diets low in sodium may reduce the risk of high blood pressure, a disease associated with many factors. A serving of __ ounces of this product conforms to such a diet.").
(B) Where the food that bears the claim is sold in a restaurant or in other establishments in which food that is ready for immediate human consumption is sold, the food can meet the requirements of paragraphs (d)(2)(vi) or (d)(2)(vii) of this section if the firm that sells the food has a reasonable basis on which to believe that the food that bears the claim meets the requirements of paragraphs (d)(2)(vi) or (d)(2)(vii) of this section and provides that basis upon request.
(3) Nutrition labeling shall be provided in the label or labeling of any food for which a health claim is made in accordance with § 101.9; for restaurant foods, in accordance with § 101.10; or for dietary supplements, in accordance with § 101.36.
(e) Prohibited health claims. No expressed or implied health claim may be made on the label or in labeling for a food, regardless of whether the food is in conventional food form or dietary supplement form, unless:
(1) The claim is specifically provided for in subpart E of this part; and
(2) The claim conforms to all general provisions of this section as well as to all specific provisions in the appropriate section of subpart E of this part;
(3) None of the disqualifying levels identified in paragraph (a)(4) of this section is exceeded in the food, unless specific alternative levels have been established for the substance in subpart E of this part; or unless FDA has permitted a claim despite the fact that a disqualifying level of a nutrient is present in the food based on a finding that such a claim will assist consumers in maintaining healthy dietary practices, and, in accordance with the regulation in subpart E of this part that makes such a finding, the label bears a disclosure statement that complies with § 101.13(h), highlighting the nutrient that exceeds the disqualifying level;
(4) Except as provided in paragraph (e)(3) of this section, no substance is present at an inappropriate level as determined in the specific provision authorizing the claim in subpart E of this part;
(5) The label does not represent or purport that the food is for infants and toddlers less than 2 years of age except if the claim is specifically provided for in subpart E of this part; and
(6) Except for dietary supplements or where provided for in other regulations in part 101, subpart E, the food contains 10 percent or more of the Reference Daily Intake or the Daily Reference Value for vitamin A, vitamin C, iron, calcium, protein, or fiber per reference amount customarily consumed prior to any nutrient addition.
(f) The requirements of this section do not apply to:
(1) Infant formulas subject to section 412(h) of the Federal Food, Drug, and Cosmetic Act, and
(2) Medical foods defined by section 5(b) of the Orphan Drug Act.
(g) Applicability. The requirements of this section apply to foods intended for human consumption that are offered for sale, regardless of whether the foods are in conventional food form or dietary supplement form.
[58 FR 2533, Jan. 6, 1993; 58 FR 17097, Apr. 1, 1993, as amended at 58 FR 44038, Aug. 18, 1993; 59 FR 425, Jan. 4, 1994; 59 FR 15050, Mar. 31, 1994; 61 FR 40332, Aug. 2, 1996; 62 FR 49867, Sept. 23, 1997; 63 FR 26980, May 15, 1998; 66 FR 17358, Mar. 30, 2001]
|
|
|
Sec. 101.15 Food; prominence of required statements.
|
|
(a) A word, statement, or other information required by or under authority of the act to appear on the label may lack that prominence and conspicuousness required by section 403(f) of the act by reason (among other reasons) of:
(1) The failure of such word, statement, or information to appear on the part or panel of the label which is presented or displayed under customary conditions of purchase;
(2) The failure of such word, statement, or information to appear on two or more parts or panels of the label, each of which has sufficient space therefor, and each of which is so designed as to render it likely to be, under customary conditions of purchase, the part or panel displayed;
(3) The failure of the label to extend over the area of the container or package available for such extension, so as to provide sufficient label space for the prominent placing of such word, statement, or information;
(4) Insufficiency of label space (for the prominent placing of such word, statement, or information) resulting from the use of label space for any word, statement, design, or device which is not required by or under authority of the act to appear on the label;
(5) Insufficiency of label space (for the prominent placing of such word, statement, or information) resulting from the use of label space to give materially greater conspicuousness to any other word, statement, or information, or to any design or device; or
(6) Smallness or style of type in which such word, statement, or information appears, insufficient background contrast, obscuring designs or vignettes, or crowding with other written, printed, or graphic matter.
(b) No exemption depending on insufficiency of label space, as prescribed in regulations promulgated under section 403 (e) or (i) of the act, shall apply if such insufficiency is caused by:
(1) The use of label space for any word, statement, design, or device which is not required by or under authority of the act to appear on the label;
(2) The use of label space to give greater conspicuousness to any word, statement, or other information than is required by section 403(f) of the act; or
(3) The use of label space for any representation in a foreign language.
(c)(1) All words, statements, and other information required by or under authority of the act to appear on the label or labeling shall appear thereon in the English language: Provided, however, That in the case of articles distributed solely in the Commonwealth of Puerto Rico or in a Territory where the predominant language is one other than English, the predominant language may be substituted for English.
(2) If the label contains any representation in a foreign language, all words, statements, and other information required by or under authority of the act to appear on the label shall appear thereon in the foreign language: Provided, however, That individual serving-size packages of foods containing no more than 1
1/2 avoirdupois ounces or no more than 1
1/2 fluid ounces served with meals in restaurants, institutions, and passenger carriers and not intended for sale at retail are exempt from the requirements of this paragraph (c)(2), if the only representation in the foreign language(s) is the name of the food.
(3) If any article of labeling (other than a label) contains any representation in a foreign language, all words, statements, and other information required by or under authority of the act to appear on the label or labeling shall appear on such article of labeling.
|
|
|
Sec. 101.17 Food labeling warning, notice, and safe handling statements.
|
|
(a) Self-pressurized containers. (1) The label of a food packaged in a self-pressurized container and intended to be expelled from the package under pressure shall bear the following warning:
WARNING - Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate. Do not store at temperature above 120 deg.F. Keep out of reach of children.
(2) In the case of products intended for use by children, the phrase "except under adult supervision" may be added at the end of the last sentence in the warning required by paragraph (a)(1) of this section.
(3) In the case of products packaged in glass containers, the word "break" may be substituted for the word "puncture" in the warning required by paragraph (a)(1) of this section.
(4) The words "Avoid spraying in eyes" may be deleted from the warning required by paragraph (a)(1) of this section in the case of a product not expelled as a spray.
(b) Self-pressurized containers with halocarbon or hydrocarbon propellants. (1) In addition to the warning required by paragraph (a) of this section, the label of a food packaged in a self-pressurized container in which the propellant consists in whole or in part of a halocarbon or a hydrocarbon shall bear the following warning:
WARNING - Use only as directed. Intentional misuse by deliberately concentrating and inhaling the contents can be harmful or fatal.
(2) The warning required by paragraph (b)(1) of this section is not required for the following products:
(i) Products expelled in the form of a foam or cream, which contain less than 10 percent propellant in the container.
(ii) Products in a container with a physical barrier that prevents escape of the propellant at the time of use.
(iii) Products of a net quantity of contents of less than 2 ounces that are designed to release a measured amount of product with each valve actuation.
(iv) Products of a net quantity of contents of less than one-half ounce.
(c) Food containing or manufactured with a chlorofluorocarbon or other ozone-depleting substance. Labeling requirements for foods that contain or are manufactured with a chlorofluorocarbon or other ozone-depleting substance designated by the Environmental Protection Agency (EPA) are set forth in 40 CFR part 82.
(d) Protein products. (1) The label and labeling of any food product in liquid, powdered, tablet, capsule, or similar forms that derives more than 50 percent of its total caloric value from either whole protein, protein hydrolysates, amino acid mixtures, or a combination of these, and that is represented for use in reducing weight shall bear the following warning:
WARNING: Very low calorie protein diets (below 400 Calories per day) may cause serious illness or death. Do Not Use for Weight Reduction in Such Diets Without Medical Supervision. Not for use by infants, children, or pregnant or nursing women.
(2) Products described in paragraph (d)(1) of this section are exempt from the labeling requirements of that paragraph if the protein products are represented as part of a nutritionally balanced diet plan providing 400 or more Calories (kilocalories) per day and the label or labeling of the product specifies the diet plan in detail or provides a brief description of that diet plan and adequate information describing where the detailed diet plan may be obtained and the label and labeling bear the following statement:
Notice: For weight reduction, use only as directed in the accompanying diet plan (the name and specific location in labeling of the diet plan may be included in this statement in place of "accompanying diet plan"). Do not use in diets supplying less than 400 Calories per day without medical supervision.
(3) The label and labeling of food products represented or intended for dietery (food) supplementation that derive more than 50 percent of their total caloric value from either whole protein, protein hydrolysates, amino acid mixtures, or a combination of these, that are represented specifically for purposes other than weight reduction; and that are not covered by the requirements of paragraph (d) (1) and (2) of this section; shall bear the following statement:
Notice: Use this product as a food supplement only. Do not use for weight reduction.
(4) The provisions of this paragraph are separate from and in addition to any labeling requirements promulgated by the Federal Trade Commission for protein supplements.
(5) Protein products shipped in bulk form for use solely in the manufacture of other foods and not for distribution to consumers in such container are exempt from the labeling requirements of this paragraph.
(6) The warning and notice statements required by paragraphs (d) (1), (2), and (3) of this section shall appear prominently and conspicuously on the principal display panel of the package label and any other labeling.
(e) Dietary supplements containing iron or iron salts. (1) The labeling of any dietary supplement in solid oral dosage form (e.g., tablets or capsules) that contains iron or iron salts for use as an iron source shall bear the following statement:
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
(2)(i) The warning statement required by paragraph (e)(1) of this section shall appear prominently and conspicuously on the information panel of the immediate container label.
(ii) If a product is packaged in unit-dose packaging, and if the immediate container bears labeling but not a label, the warning statement required by paragraph (e)(1) of this section shall appear prominently and conspicuously on the immediate container labeling in a way that maximizes the likelihood that the warning is intact until all of the dosage units to which it applies are used.
(3) Where the immediate container is not the retail package, the warning statement required by paragraph (e)(1) of this section shall also appear prominently and conspicuously on the information panel of the retail package label.
(4) The warning statement shall appear on any labeling that contains warnings.
(5) The warning statement required by paragraph (e)(1) of this section shall be set off in a box by use of hairlines.
(f) Foods containing psyllium husk. (1) Foods containing dry or incompletely hydrated psyllium husk, also known as psyllium seed husk, and bearing a health claim on the association between soluble fiber from psyllium husk and reduced risk of coronary heart disease, shall bear a label statement informing consumers that the appropriate use of such foods requires consumption with adequate amounts of fluids, alerting them of potential consequences of failing to follow usage recommendations, and informing persons with swallowing difficulties to avoid consumption of the product (e.g., "NOTICE: This food should be eaten with at least a full glass of liquid. Eating this product without enough liquid may cause choking. Do not eat this product if you have difficulty in swallowing."). However, a product in conventional food form may be exempt from this requirement if a viscous adhesive mass is not formed when the food is exposed to fluids.
(2) The statement shall appear prominently and conspicuously on the information panel or principal display panel of the package label and any other labeling to render it likely to be read and understood by the ordinary individual under customary conditions of purchase and use. The statement shall be preceded by the word "NOTICE" in capital letters.
(g) Juices that have not been specifically processed to prevent, reduce, or eliminate the presence of pathogens. (1) For purposes of this paragraph (g), "juice" means the aqueous liquid expressed or extracted from one or more fruits or vegetables, purees of the edible portions of one or more fruits or vegetables, or any concentrate of such liquid or puree.
(2) The label of:
(i) Any juice that has not been processed in the manner described in paragraph (g)(7) of this section; or
(ii) Any beverage containing juice where neither the juice ingredient nor the beverage has been processed in the manner described in paragraph (g)(7) of this section, shall bear the following warning statement:
WARNING: This product has not been pasteurized and, therefore, may contain harmful bacteria that can cause serious illness in children, the elderly, and persons with weakened immune systems.
(3) The warning statement required by this paragraph (g) shall not apply to juice that is not for distribution to retail consumers in the form shipped and that is for use solely in the manufacture of other foods or that is to be processed, labeled, or repacked at a site other than originally processed, provided that for juice that has not been processed in the manner described in paragraph (g)(7) of this section, the lack of such processing is disclosed in documents accompanying the juice, in accordance with the practice of the trade.
(4) The warning statement required by paragraph (g)(2) of this section shall appear prominently and conspicuously on the information panel or on the principal display panel of the label of the container.
(5) The word "WARNING" shall be capitalized and shall appear in bold type.
(6) The warning statement required by paragraph (g)(2) of this section, when on a label, shall be set off in a box by use of hairlines.
(7)(i) The requirements in this paragraph (g) shall not apply to a juice that has been processed in a manner that will produce, at a minimum, a reduction in the pertinent microorganism for a period at least as long as the shelf life of the product when stored under normal and moderate abuse conditions, of the following magnitude:
(A) A 5-log (i.e., 100,000-fold) reduction; or
(B) A reduction that is equal to, or greater than, the criterion established for process controls by any final regulation requiring the application of Hazard Analysis and Critical Control Point (HACCP) principles to the processing of juice.
(ii) For the purposes of this paragraph (g), the "pertinent microorganism" is the most resistant microorganism of public health significance that is likely to occur in the juice.
(h) Shell eggs. (1) The label of all shell eggs, whether in intrastate or interstate commerce, shall bear the following statement:
SAFE HANDLING INSTRUCTIONS: To prevent illness from bacteria: keep eggs refrigerated, cook eggs until yolks are firm, and cook foods containing eggs thoroughly.
(2) The label statement required by paragraph (h)(1) of this section shall appear prominently and conspicuously, with the words "SAFE HANDLING INSTRUCTIONS" in bold type, on the principal display panel, the information panel, or on the inside of the lid of egg cartons. If this statement appears on the inside of the lid, the words "Keep Refrigerated" must appear on the principal display panel or information panel.
(3) The label statement required by paragraph (h)(1) of this section shall be set off in a box by use of hairlines.
(4) Shell eggs that have been, before distribution to consumers, specifically processed to destroy all viable Salmonella shall be exempt from the requirements of paragraph (h) of this section.
(5) The safe handling statement for shell eggs that are not for direct sale to consumers, e.g., those that are to be repacked or labeled at a site other than where originally processed, or are sold for use in food service establishments, may be provided on cartons or in labeling, e.g., invoices or bills of lading in accordance with the practice of the trade.
(6) Under sections 311 and 361 of the Public Health Service Act (PHS Act), any State or locality that is willing and able to assist the agency in the enforcement of paragraphs (h)(1) through (h)(5) of this section, and is authorized to inspect or regulate establishments handling packed shell eggs, may in its own jurisdiction, enforce paragraphs (h)(1) through (h)(5) of this section through inspections under paragraph (h)(8) of this section and through administrative enforcement remedies identified in paragraph (h)(7) of this section until FDA notifies the State or locality in writing that such assistance is no longer needed. When providing such assistance, a State or locality may follow the hearing procedures set out in paragraphs (h)(7)(ii)(C) through (h)(7)(ii)(D) of this section, substituting, where necessary, appropriate State or local officials for designated FDA officials or may utilize State or local hearing procedures if such procedures satisfy due process.
(7) This paragraph (h) is established under authority of both the Federal Food, Drug, and Cosmetic Act (the act) and the PHS Act. Under the act, the agency can enforce the food misbranding provisions under 21 U.S.C. 331, 332, 333, and 334. However, 42 U.S.C. 264 provides for the issuance of implementing enforcement regulations; therefore, FDA has established the following administrative enforcement procedures for the relabeling, diversion, or destruction of shell eggs and informal hearings under the PHS Act:
(i) Upon finding that any shell eggs are in violation of this section an authorized FDA representative or State or local representative in accordance with paragraph (h)(6) of this section may order such eggs to be relabeled under the supervision of said representative, diverted, under the supervision of said representative for processing in accordance with the Egg Products Inspection Act (EPIA) (21 U.S.C. 1031 et seq. ), or destroyed by or under the supervision of an officer or employee of the FDA, or, if applicable, of the State or locality, in accordance with the following procedures:
(A) Order for relabeling, diversion, or destruction under the PHS Act. Any division office of FDA or any State or locality acting under paragraph (h)(6) of this section, upon finding shell eggs held in violation of this section, may serve upon the person in whose possession such eggs are found a written order that such eggs be relabeled with the required statement in paragraph (h)(1) of this section before further distribution. If the person chooses not to relabel, the division office of FDA or, if applicable, the appropriate State or local agency may serve upon the person a written order that such eggs be diverted (from direct consumer sale, e.g., to food service) under the supervision of an officer or employee of the issuing entity, for processing in accordance with the EPIA (21 U.S.C. 1031 et seq. ) or destroyed by or under the supervision of the issuing entity, within 10 working days from the date of receipt of the order.
(B) Issuance of order. The order shall include the following information:
(1 ) A statement that the shell eggs identified in the order are subject to relabeling, diversion for processing in accordance with the EPIA, or destruction;
(2 ) A detailed description of the facts that justify the issuance of the order;
(3 ) The location of the eggs;
(4 ) A statement that these eggs shall not be sold, distributed, or otherwise disposed of or moved except as provided in paragraph (h)(7)(i)(E) of this section;
(5 ) Identification or description of the eggs;
(6 ) The order number;
(7 ) The date of the order;
(8 ) The text of this entire section;
(9 ) A statement that the order may be appealed by written appeal or by requesting an informal hearing;
(10 ) The name and phone number of the person issuing the order; and
(11 ) The location and telephone number of the responsible office or agency and the name of its director.
(C) Approval of director. An order, before issuance, shall be approved by the director of the office or agency issuing the order. If prior written approval is not feasible, prior oral approval shall be obtained and confirmed by written memorandum as soon as possible.
(D) Labeling or marking of shell eggs under order. An FDA, State, or local representative issuing an order under paragraph (h)(7)(i)(A) of this section shall label or mark the shell eggs with official tags that include the following information:
(1 ) A statement that the shell eggs are detained in accordance with regulations issued under section 361(a) of the PHS Act (42 U.S.C. 264(a)).
(2 ) A statement that the shell eggs shall not be sold, distributed or otherwise disposed of or moved except, after notifying the issuing entity in writing, to:
(i ) Relabel, divert them for processing in accordance with the EPIA, or destroy them, or
(ii ) Move them to another location for holding pending appeal.
(3 ) A statement that the violation of the order or the removal or alteration of the tag is punishable by fine or imprisonment or both (section 368 of the PHS Act, 42 U.S.C. 271).
(4 ) The order number and the date of the order, and the name of the government representative who issued the order.
(E) Sale or other disposition of shell eggs under order. After service of the order, the person in possession of the shell eggs that are the subject of the order shall not sell, distribute, or otherwise dispose of or move any eggs subject to the order unless and until the notice is withdrawn after an appeal except, after notifying FDA's division office or, if applicable, the State or local agency in writing, to:
(1 ) Relabel, divert, or destroy them as specified in paragraph (h)(7)(i) of this section, or
(2 ) Move them to another location for holding pending appeal.
(ii) The person on whom the order for relabeling, diversion, or destruction is served may either comply with the order or appeal the order to an Office of Regulatory Affairs Program Director.
(A) Appeal of a detention order. Any appeal shall be submitted in writing to the FDA Division Director in whose division the shell eggs are located within 5 working days of the issuance of the order. If the appeal includes a request for an informal hearing, the hearing shall be held within 5 working days after the appeal is filed or, if requested by the appellant, at a later date, which shall not be later than 20 calendar days after the issuance of the order. The order may also be appealed within the same period of 5 working days by any other person having an ownership or proprietary interest in such shell eggs. The appellant of an order shall state the ownership or proprietary interest the appellant has in the shell eggs.
(B) Summary decision. A request for a hearing may be denied, in whole or in part and at any time after a request for a hearing has been submitted, if the Office of Regulatory Affairs Program Director or another FDA official senior to an FDA Division Director determines that no genuine and substantial issue of fact has been raised by the material submitted in connection with the hearing or from matters officially noticed. If the presiding FDA official determines that a hearing is not justified, written notice of the determination will be given to the parties explaining the reason for denial.
(C) Informal hearing. Appearance by any appellant at the hearing may be by mail or in person, with or without counsel. The informal hearing shall be conducted by an Office of Regulatory Affairs Program Director or another FDA official senior to an FDA Division Director, and a written summary of the proceedings shall be prepared by the presiding FDA official.
(1 ) The presiding FDA official may direct that the hearing be conducted in any suitable manner permitted by law and this section. The presiding FDA official has the power to take such actions and make such rulings as are necessary or appropriate to maintain order and to conduct an informal, fair, expeditious, and impartial hearing, and to enforce the requirements concerning the conduct of hearings.
(2 ) Employees of FDA will first give a full and complete statement of the action which is the subject of the hearing, together with the information and reasons supporting it, and may present oral or written information relevant to the hearing. The party requesting the hearing may then present oral or written information relevant to the hearing. All parties may conduct reasonable examination of any person (except for the presiding officer and counsel for the parties) who makes any statement on the matter at the hearing.
(3 ) The hearing shall be informal in nature, and the rules of evidence do not apply. No motions or objections relating to the admissibility of information and views will be made or considered, but any party may comment upon or rebut any information and views presented by another party.
(4 ) The party requesting the hearing may have the hearing transcribed, at the party's expense, in which case a copy of the transcript is to be furnished to FDA. Any transcript of the hearing will be included with the presiding FDA official's report of the hearing.
(5 ) The presiding FDA official shall prepare a written report of the hearing. All written material presented at the hearing will be attached to the report. Whenever time permits, the presiding FDA official may give the parties the opportunity to review and comment on the report of the hearing.
(6 ) The presiding FDA official shall include as part of the report of the hearing a finding on the credibility of witnesses (other than expert witnesses) whenever credibility is a material issue, and shall include a recommended decision, with a statement of reasons.
(D) Written appeal. If the appellant appeals the detention order but does not request a hearing, the presiding FDA official shall render a decision on the appeal affirming or revoking the detention within 5-working days after the receipt of the appeal.
(E) Presiding FDA official's decision. If, based on the evidence presented at the hearing or by the appellant in a written appeal, the presiding FDA official finds that the shell eggs were held in violation of this section, he shall affirm the order that they be relabeled, diverted under the supervision of an officer or employee of FDA for processing under the EPIA, or destroyed by or under the supervision of an officer or employee of FDA; otherwise, the presiding FDA official shall issue a written notice that the prior order is withdrawn. If the presiding FDA official affirms the order, he shall order that the relabeling, diversion, or destruction be accomplished within 10-working days from the date of the issuance of his decision. The presiding FDA official's decision shall be accompanied by a statement of the reasons for the decision. The decision of the presiding FDA official shall constitute final agency action, reviewable in the courts.
(F) No appeal. If there is no appeal of the order and the person in possession of the shell eggs that are subject to the order fails to relabel, divert, or destroy them within 10 working days, or if the demand is affirmed by the presiding FDA official after an appeal and the person in possession of such eggs fails to relabel, divert, or destroy them within 10 working days, the FDA division office, or, if applicable, the State or local agency may designate an officer or employee to divert or destroy such eggs. It shall be unlawful to prevent or to attempt to prevent such diversion or destruction of the shell eggs by the designated officer or employee.
(8) Persons engaged in handling or storing packed shell eggs for retail distribution shall permit authorized representatives of FDA to make at any reasonable time such inspection of the establishment in which shell eggs are being held, including inspection and sampling of the labeling of such eggs as may be necessary in the judgment of such representatives to determine compliance with the provisions of this section. Inspections may be made with or without notice and will ordinarily be made during regular business hours.
(9) No State or local governing entity shall establish or continue in effect any law, rule, regulation, or other requirement requiring safe handling instructions on unpasteurized shell eggs that are less stringent than those required in paragraphs (h)(1) through (h)(5) of this section.
[42 FR 14308, Mar. 15, 1977] Editorial Note: For Federal Register citations affecting § 101.17, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and at www.govinfo.gov.
|
|
|
Sec. 101.18 Misbranding of food.
|
|
(a) Among representations in the labeling of a food which render such food misbranded is a false or misleading representation with respect to another food or a drug, device, or cosmetic.
(b) The labeling of a food which contains two or more ingredients may be misleading by reason (among other reasons) of the designation of such food in such labeling by a name which includes or suggests the name of one or more but not all such ingredients, even though the names of all such ingredients are stated elsewhere in the labeling.
(c) Among representations in the labeling of a food which render such food misbranded is any representation that expresses or implies a geographical origin of the food or any ingredient of the food except when such representation is either:
(1) A truthful representation of geographical origin.
(2) A trademark or trade name provided that as applied to the article in question its use is not deceptively misdescriptive. A trademark or trade name composed in whole or in part of geographical words shall not be considered deceptively misdescriptive if it:
(i) Has been so long and exclusively used by a manufacturer or distributor that it is generally understood by the consumer to mean the product of a particular manufacturer or distributor; or
(ii) Is so arbitrary or fanciful that it is not generally understood by the consumer to suggest geographic origin.
(3) A part of the name required by applicable Federal law or regulation.
(4) A name whose market significance is generally understood by the consumer to connote a particular class, kind, type, or style of food rather than to indicate geographical origin.
|
|
|
Appendix A to Part 101 [Reserved]
|
|
|
|
|
Appendix B to Part 101 - Graphic Enhancements Used by the FDA
|
|




[83 FR 65504, Dec. 21, 2018]
|
|
|
Appendix C to Part 101 - Nutrition Facts for Raw Fruits and Vegetables
|
|
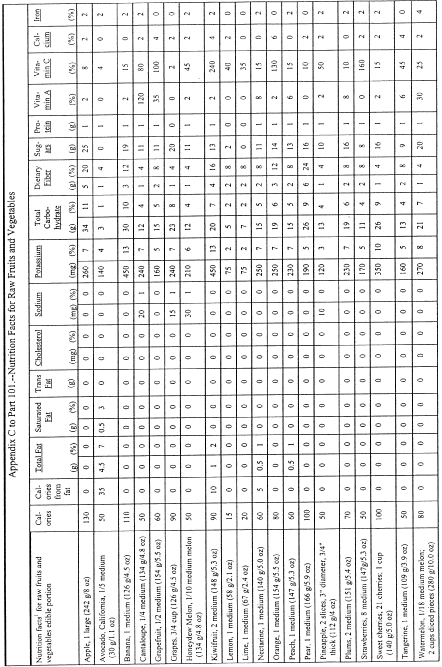
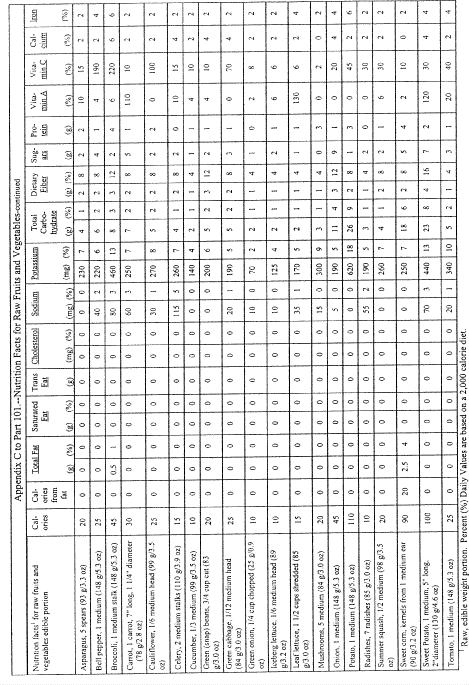


[71 FR 47439, Aug. 17, 2006]
|
|
|
Appendix D to Part 101 - Nutrition Facts for Cooked Fish
|
|
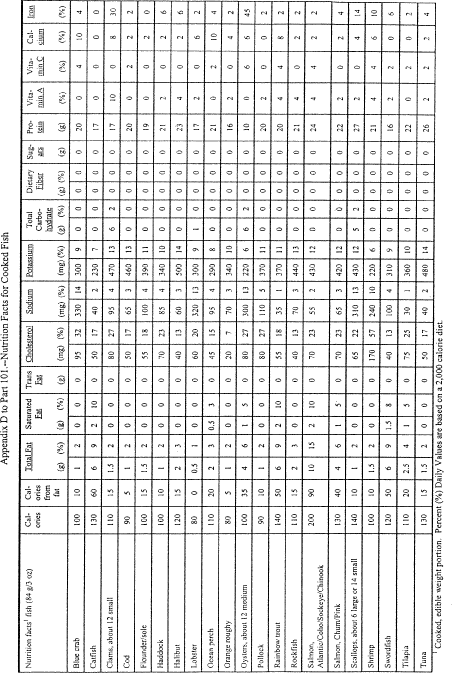

[71 FR 47439, Aug. 17, 2006]
|
|
|
Authority: 15 U.S.C. 1453, 1454, 1455; 21 U.S.C. 321, 331, 342, 343, 348, 371; 42 U.S.C. 243, 264, 271.
Source: 42 FR 14308, Mar. 15, 1977, unless otherwise noted.
|
|