|
|
| [Code of Federal Regulations] |
| [Title 21, Volume 8] |
| [CITE: 21CFR1230] |
TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUBCHAPTER L - REGULATIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRATION
| |
| PART 1230 | REGULATIONS UNDER THE FEDERAL CAUSTIC POISON ACT |
|
|
|
|
Subpart A - General Provisions
|
|
Sec. 1230.2 Scope of the act.
|
|
The provisions of the act apply to any container which has been shipped or delivered for shipment in interstate or foreign commerce, as defined in section 2(c) of the act (44 Stat. 1407; 15 U.S.C. 402) or which has been received from shipment in such commerce for sale or exchange, or which is sold or offered for sale or held for sale or exchange in any Territory or possession or in the District of Columbia.
|
|
|
(a) The word container as used in the regulations in this part means a retail parcel, package, or container suitable for household use and employed exclusively to hold any dangerous caustic or corrosive substance defined in the act.
(b) The words suitable for household use mean and imply adaptability for ready or convenient handling in places where people dwell.
|
|
|
Subpart B - Labeling
|
|
The label or sticker shall be so firmly attached to the container that it will remain thereon while the container is being used, and be so placed as readily to attract attention.
|
|
|
Sec. 1230.11 Required wording.
|
|
(a) The common name of the dangerous caustic or corrosive substance which shall appear on the label or sticker is the name given in section 2(a) of the act (44 Stat. 1406; 15 U.S.C. 402) or any other name commonly employed to designate and identify such substance.
(b) Preparations within the scope of the act bearing trade or fanciful names shall, in addition, be labeled with the common name of the dangerous caustic or corrosive substance contained therein and comply with all the other requirements of the act and of the regulations in this part.
|
|
|
Sec. 1230.12 Manufacturer; distributor.
|
|
If the name on the label or sticker is other than that of the manufacturer, it shall be qualified by such words as "packed for," "packed by," "sold by," or "distributed by," as the case may be, or by other appropriate expression.
|
|
|
Sec. 1230.13 Labeling of "poison".
|
|
The following are styles of uncondensed Gothic capital letters 24-point (type face) size:
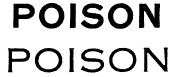
When letters of not less than 24-point size are required on a label in stating the word "poison" they must not be smaller than those above set forth.
|
|
|
Sec. 1230.14 Directions for treatment.
|
|
Except as provided in § 1230.16, the container shall bear in all cases upon the label or sticker thereof, immediately following the word "Poison," directions for treatment in the case of internal personal injury; in addition, if the substance may cause external injury, directions for appropriate treatment shall be given. The directions shall prescribe such treatments for personal injury as are sanctioned by competent medical authority, and the materials called for by such directions shall be, whenever practicable, such as are usually available in the household.
|
|
|
Sec. 1230.15 Responsibility for labeling directions for treatment.
|
|
A person who receives from a manufacturer or wholesaler any container which under the conditions set forth in section 2(b)(4) of the act and § 1230.16 does not bear at the time of shipment directions for treatment in the case of personal injury must place such directions on the label or sticker if he offers such container for general sale or exchange.
|
|
|
Sec. 1230.16 Exemption from labeling directions for treatment.
|
|
Manufacturers and wholesalers only, at the time of shipment or delivery for shipment, are exempted from placing directions for treatment on the label or sticker of any container for other than household use, but in any event the information required by section 2(b) (1), (2), and (3) of the act (44 Stat. 1407; 15 U.S.C. 402) and the regulations in this part shall be given.
|
|
|
Subpart C - Guaranty
|
|
Sec. 1230.20 General guaranty.
|
|
In lieu of a particular guaranty for each lot of dangerous caustic or corrosive substances, a general continuing guaranty may be furnished by the guarantor to actual or prospective purchasers. The following are forms of continuing guaranties:
(a) Substances for both household use and other than household use:
The undersigned guarantees that the retail parcels, packages, or containers of the dangerous caustic or corrosive substance or substances to be sold to _____ are not misbranded within the meaning of the Federal Caustic Poison Act.
(Date)
(Signature and address of
guarantor)
(b) Substances for other than household use (this form may be issued only by a manufacturer or wholesaler) (§§ 1230.15, 1230.16):
The dangerous caustic or corrosive substance or substances in retail parcels, packages, or containers suitable for household use to be sold to _____ are for other than household use, and guaranteed not to be misbranded within the meaning of the Federal Caustic Poison Act.
(Date)
(Signature and address of
manufacturer or wholesaler)
|
|
|
Sec. 1230.21 Specific guaranty.
|
|
If a guaranty in respect to any specific lot of dangerous caustic or corrosive substances be given, it shall be incorporated in or attached to the bill of sale, invoice, or other schedule bearing the date and the name and quantity of the substance sold, and shall not appear on the label or package. The following are forms of specific guaranties:
(a) Substances for both household use and other than household use:
The undersigned guarantees that the retail parcels, packages, or containers of the dangerous caustic or corrosive substance or substances listed herein (or specifying the substances) are not misbranded within the meaning of the Federal Caustic Poison Act.
(Signature and address of guarantor)
(b) Substances for other than household use (this form may be issued only by a manufacturer or wholesaler (§§ 1230.15, 1230.16):
The dangerous caustic or corrosive substance or substances listed herein (or specifying the substances) in retail parcels, packages, or containers suitable for household use are for other than household use and are guaranteed not to be misbranded within the meaning of the Federal Caustic Poison Act.
(Name and address of manufacturer
or wholesaler)
|
|
|
Subpart D - Administrative Procedures
|
|
Sec. 1230.30 Collection of samples.
|
|
Samples for examination by or under the direction and supervision of the Food and Drug Administration shall be collected by:
(a) An authorized agent in the employ of the Department of Health and Human Services;
(b) Any officer of any State, Territory, or possession, or of the District of Columbia, authorized by the Secretary of Health and Human Services.
|
|
|
Sec. 1230.31 Where samples may be collected.
|
|
Caustic or corrosive substances within the scope of this act (44 Stat. 1406; 15 U.S.C. 401-411) may be sampled wherever found.
|
|
|
Sec. 1230.32 Analyzing of samples.
|
|
Samples collected by an authorized agent shall be analyzed at the laboratory designated by the Food and Drug Administration. Only such samples as are collected in accordance with §§ 1230.30, 1230.31 may be analyzed by or under the direction and supervision of the Food and Drug Administration. Upon request one subdivision of the sample, if available, shall be delivered to the party or parties interested.
|
|
|
Sec. 1230.33 Investigations.
|
|
Authorized agents in the employ of the Department of Health and Human Services may make investigations, including the inspection of premises where dangerous caustic and corrosive substances subject to the act are manufactured, packed, stored, or held for sale or distribution, and make examinations of freight and other transportation records.
|
|
|
(a) The methods of examination or analysis employed shall be those prescribed by the Association of Official Agricultural Chemists, when applicable, provided, however, that any method of analysis or examination satisfactory to the Food and Drug Administration may be employed.
(b) All percentages stated in the definitions in section 2(a) of the Federal Caustic Poison Act shall be determined by weight.
|
|
|
Whenever it appears from the inspection, analysis, or test of any container that the provisions of section 3 or 6 of the Federal Caustic Poison Act (44 Stat. 1407, 1409; 15 U.S.C. 403, 406) have been violated and criminal proceedings are contemplated, notice shall be given to the party or parties against whom prosecution is under consideration and to other interested parties, and a date shall be fixed at which such party or parties may be heard. The hearing shall be held at the office of the Food and Drug Administration designated in the notice and shall be private and confined to questions of fact. The parties notified may present evidence, either oral or written, in person or by attorney, to show cause why the matter should not be referred for prosecution as a violation of the Federal Caustic Poison Act.
|
|
|
Sec. 1230.36 Hearings; when not provided for.
|
|
No hearing is provided for when the health, medical, or drug officer or agent of any State, Territory, or possession, or of the District of Columbia, acts under the authority contained in section 8 of the Federal Caustic Poison Act (44 Stat. 1409; 15 U.S.C. 408) in reporting a violation direct to the United States attorney.
|
|
|
Sec. 1230.37 Publication.
|
|
(a) After judgment of the court in any proceeding under the Federal Caustic Poison Act, notice shall be given by publication. Such notice shall include the findings of the court and may include the findings of the analyst and such explanatory statements of fact as the Secretary of Health and Human Services may deem appropriate.
(b) This publication may be made in the form of a circular, notice, or bulletin, as the Secretary of Health and Human Services may direct.
(c) If an appeal be taken from the judgment of the court before such publication, that fact shall appear.
|
|
|
Subpart E - Imports
|
|
Sec. 1230.40 Required label information.
|
|
Containers which are offered for import shall in all cases bear labels or stickers having thereon the information required by section 2(b) (1), (2), and (3) of the Federal Caustic Poison Act and the directions for treatment in the case of personal injury, except such directions need not appear on the label or sticker at the time of shipment by a wholesaler or manufacturer for other than household use.
|
|
|
Sec. 1230.41 Delivery of containers.
|
|
Containers shall not be delivered to the consignee prior to report of examination, unless a bond has been given on the appropriate form for the amount of the full invoice value of such containers, together with the duty thereon, and the refusal of the consignee to return such containers for any cause to the custody of the District Director of Customs when demanded, for the purpose of excluding them from the country or for any other purpose, the consignee shall pay an amount equal to the sum named in the bond, and such part of the duty, if any, as may be payable, as liquidated damages for failure to return to the District Director of Customs on demand all containers covered by the bond.
|
|
|
As soon as the importer makes entry, the invoices covering containers and the public stores packages shall be made available, with the least possible delay, for inspection by the representative of the district. When no sample is desired the invoice shall be stamped by the district "No sample desired, Food and Drug Administration, Department of Health and Human Services, per (initials of inspecting officer)."
|
|
|
Sec. 1230.43 Enforcement.
|
|
(a) Enforcement agency. The Federal Caustic Poison Act shall be enforced by the Food and Drug Administration, Department of Health and Human Services.
(b) Enforcement of provisions. The enforcement of the provisions of the Federal Caustic Poison Act as they relate to imported dangerous caustic or corrosive substances, will, as a general rule, be under the direction of the chief of the local inspection district of the Food and Drug Administration, Department of Health and Human Services, and District Directors of Customs acting as administrative officers in carrying out directions relative to the detention, exportation, and sale, or other disposition of such substances and action under the bond in case of noncompliance with the provisions of the Federal Caustic Poison Act.
(c) Chief of district as customs officer. The chief of district shall be deemed a customs officer in enforcing import regulations.
(d) Nonlaboratory ports. (1) At the ports of entry where there is no district of the Food and Drug Administration, the District Director of Customs or deputy, on the day when the first notice of expected shipment of containers is received, either by invoice or entry, shall notify the chief of district in whose territory the port is located.
(2) On the day of receipt of such notice the chief of district shall mail to the District Director of Customs appropriate notice, if no sample is desired. This notice serves as an equivalent to stamping the invoices at district ports with the legend "No sample desired, Food and Drug Administration, Department of Health and Human Services, per (initials of inspecting officer)."
(3) If samples are desired, the Chief of district shall immediately notify the District Director of Customs.
(4) The District Director of Customs at once shall forward samples, accompanied by description of shipment.
(5) When samples are desired from each shipment of containers, the chief of district shall furnish to District Director of Customs and deputies at ports within the district's territory a list of such containers, indicating the size of sample necessary. Samples should then be sent promptly on arrival of containers without awaiting special request.
(6) In all other particulars the procedure shall be the same at nonlaboratory ports as at laboratory ports, except that the time consumed in delivery of notices by mail shall be allowed for.
|
|
|
On the same day that samples are requested by the district, the District Director of Customs or appraiser shall notify the importer that samples will be taken, that the containers must be held intact pending a notice of the result of inspection and analysis, and that in case the containers do not comply with the requirements of the Federal Caustic Poison Act, they must be returned to the District Director of Customs for disposition. This notification may be given by the District Director of Customs or appraiser through individual notices to the importer or by suitable bulletin notices posted daily in the customhouse.
|
|
|
Sec. 1230.45 No violation; release.
|
|
As soon as examination of the samples is completed, if no violation of the act is detected, the chief of the district shall send a notice of release to the importer and a copy of this notice to the District Director of Customs for his information.
|
|
|
(a) If a violation of the Federal Caustic Poison Act is disclosed, the chief of the district shall send to the importer due notice of the nature of the violation and of the time and place where evidence may be presented, showing that the containers should not be refused admission. At the same time similar notice regarding detention of the containers shall be sent to the District Director of Customs, requesting him to refuse delivery thereof or to require their return to customs custody if by any chance the containers were released without the bond referred to in § 1230.41. The time allowed the importer for representations regarding the shipment may be extended at his request for a reasonable period to permit him to secure such evidence.
(b) If the importer does not reply to the notice of hearing in person or by letter within the time allowed on the notice, a second notice, marked "second and last notice," shall be sent at once by the chief of the district, advising him that failure to reply will cause definite recommendation to the District Director of Customs that the containers be refused admission and that the containers be exported within 3 months under customs supervision.
|
|
|
Sec. 1230.47 Rejected containers.
|
|
(a) In all cases where the containers are to be refused admission, the chief of the district within 1 day after hearing, or, if the importer does not appear or reply within 3 days after second notice, shall notify the District Director of Customs in duplicate accordingly.
(b) Not later than 1 day after receipt of this notice the District Director of Customs shall sign and transmit to the importer one of the copies, which shall serve as notification to the importer that the containers must be exported under customs supervision within 3 months from such date, as provided by law; the other notice shall be retained as office record and later returned as a report to the chief of the district. In all cases the importer shall return his notice to the District Director of Customs, properly certified as to the information required, as the form provides.
|
|
|
Sec. 1230.48 Relabeling of containers.
|
|
(a) If containers are to be released after relabeling, a notice shall be sent by the chief of district direct to the importer, a carbon copy being sent to the District Director of Customs. This notice must state specifically the conditions to be performed, so as to bring the performance thereof under the provisions of the customs bonds on consumption and warehouse entries, these bonds including provisions requiring compliance with all of the requirements of the Federal Caustic Poison Act and all regulations and instructions issued thereunder. The notice will also state the officer to be notified by the importer when the containers are ready for inspection.
(b) The importer must return the notice to the District Director of Customs or chief of district, as designated, with the certificate thereon filled out, stating that he has complied with the prescribed conditions and that the containers are ready for inspection at the place named.
(c) This notice will be delivered to the inspection officer, who, after inspection, will endorse the result thereof on the back of the notice and return the same to the District Director of Customs or to the chief of district, as the case may be.
(d) When the conditions to be complied with are under the supervision of the chief of district, and these conditions have been fully met, he shall release the containers to the importer, sending a copy of the notice of release to the District Director of Customs for his information. If the containers have not been properly relabeled within the period allowed, the chief of district shall immediately give notice in duplicate to the District Director of Customs of the results of inspection. The District Director of Customs shall sign and immediately transmit one copy of the notice to the importer and proceed in the usual manner.
(e) If the containers are detained subject to relabeling to be performed under the supervision of the District Director of Customs, the District Director of Customs, as soon as relabeling is accomplished, will notify the importer that the containers are released.
(f) If the containers have not been properly relabeled within the period allowed, their sale after labeling as required by the act or other disposition must be effected by the District Director of Customs.
(g) When the final action has been taken on containers which have been refused admission, sold, or otherwise disposed of as provided for by the act or which have been relabeled under the supervision of the District Director of Customs, he shall send to the chief of district a notice of such final action, giving the date and disposition.
(h) When relabeling is allowed the importer must furnish satisfactory evidence as to the identity of the containers before release is given. The relabeling must be done at a stated place and apart from other containers of a similar nature.
(i) When containers are shipped to another port for relabeling or exportation, they must be shipped under customs carrier's manifest, in the same manner as shipments in bond.
(j) District Directors of Customs will perform the inspection service whenever containers are to be exported, sold, or otherwise disposed of, and in other cases when there is no officer of the district available.
(k) District Directors of Customs and representatives of the district will confer and arrange the apportionment of the inspection service according to local conditions. Officers of the district will, whenever feasible, perform the inspection service in connection with relabeling.
|
|
|
(a) In case of failure to comply with the instructions or recommendations of the chief of district as to conditions under which containers may be disposed of, the District Director of Customs shall notify the chief of district in all cases coming to his attention within 3 days after inspection or after the expiration of the 3 months allowed by law if no action is taken.
(b) The chief of district, upon receipt of the above-described notice, and in all cases of failure to meet the conditions imposed in order to comply with the provisions of the Federal Caustic Poison Act coming directly under his supervision, shall transmit to the District Director of Customs such evidence as he may have at hand tending to indicate the importer's liability and make a recommendation accordingly.
(c) The District Director of Customs, within 3 days of the receipt of this recommendation, whether favorable or otherwise, shall notify the importer that, the legal period of 3 months for exportation or relabeling having expired, action will be taken within 30 days to enforce the terms of the bond.
|
|
|
Authority: 15 U.S.C. 1261-1276.
Source: 38 FR 32110, Nov. 20, 1973, unless otherwise noted.
|
|