|
Semirigid and rigid acrylic and modified acrylic plastics may be safely used as articles intended for use in contact with food, in accordance with the following prescribed conditions. The acrylic and modified acrylic polymers or plastics described in this section also may be safely used as components of articles intended for use in contact with food.
(a) The optional substances that may be used in the formulation of the semirigid and rigid acrylic and modified acrylic plastics, or in the formulation of acrylic and modified acrylic components of articles, include substances generally recognized as safe in food, substances used in accordance with a prior sanction or approval, substances permitted for use in such plastics by regulations in parts 170 through 189 of this chapter, and substances identified in this paragraph. At least 50 weight-percent of the polymer content of the acrylic and modified acrylic materials used as finished articles or as components of articles shall consist of polymer units derived from one or more of the acrylic or methacrylic monomers listed in paragraph (a)(1) of this section.
(1) Homopolymers and copolymers of the following monomers:
n- Butyl acrylate.
n- Butyl methacrylate.
Ethyl acrylate.
2-Ethylhexyl acrylate.
Ethyl methacrylate.
Methyl acrylate.
Methyl methacrylate.
(2) Copolymers produced by copolymerizing one or more of the monomers listed in paragraph (a)(1) of this section with one or more of the following monomers:
Acrylonitrile.
Methacrylonitrile.
[alpha]-Methylstyrene.
Styrene.
Vinyl chloride.
Vinylidene chloride.
(3) Polymers identified in paragraphs (a)(1) and (2) of this section containing no more than 5 weight-percent of total polymer units derived by copolymerization with one or more of the monomers listed in paragraph (a)(3)(i) and (ii) of this section. Monomers listed in paragraph (a)(3)(ii) of this section are limited to use only in plastic articles intended for repeated use in contact with food.
(i) List of minor monomers:
Acrylamide.
Acrylic acid
1,3-Butylene glycol dimethacrylate.
1,4-Butylene glycol dimethacrylate.
Diethylene glycol dimethacrylate.
Diproplylene glycol dimethacrylate.
Divinylbenzene.
Ethylene glycol dimethacrylate.
Itaconic acid.
Methacrylic acid.
N- Methylolacrylamide.
N- Methylolmethacrylamide.
4-Methyl-1,4-pentanediol dimethacrylate.
Propylene glycol dimethacrylate.
Trivinylbenzene.
(ii) List of minor monomers limited to use only in plastic articles intended for repeated use in contact with food:
Allyl methacrylate [Chemical Abstracts Service Registry No. 96-05-9]
tert- Butyl acrylate.
tert- Butylaminoethyl methacrylate.
sec- Butyl methacrylate.
tert- Butyl methacrylate.
Cyclohexyl methacrylate.
Dimethylaminoethyl methacrylate.
2-Ethylhexyl methacrylate.
Hydroxyethyl methacrylate.
Hydroxyethyl vinyl sulfide.
Hydroxypropyl methacrylate.
Isobornyl methacrylate.
Isobutyl methacrylate.
Isopropyl acrylate.
Isopropyl methacrylate.
Methacrylamide.
Methacrylamidoethylene urea.
Methacryloxyacetamidoethylethylene urea.
Methacryloxyacetic acid.
n- Propyl methacrylate.
3,5,5-Trimethylcyclohexyl methacrylate.
(4) Polymers identified in paragraphs (a)(1), (2), and (3) of this section are mixed together and/or with the following polymers, provided that no chemical reactions, other than addition reactions, occur when they are mixed:
Butadiene-acrylonitrile copolymers.
Butadiene-acrylonitrile-styrene copolymers.
Butadiene-acrylonitrile-styrene-methyl methacrylic copolymers.
Butadiene-styrene copolymers.
Butyl rubber.
Natural rubber.
Polybutadiene.
Poly (3-chloro-1,3-butadiene).
Polyester identified in § 175.300(b)(3)(vii) of this chapter.
Polyvinyl chloride.
Vinyl chloride copolymers complying with § 177.1980.
Vinyl chloride-vinyl acetate copolymers.
(5) Antioxidants and stabilizers identified in § 175.300(b)(3)(xxx) of this chapter and the following:
Di-tert- butyl-p- cresol.
2-Hydroxy-4-methoxybenzophenone.
2-Hydroxy-4-methoxy-2-carboxybenzophenone.
3-Hydroxyphenyl benzoate.
p- Methoxyphenol.
Methyl salicylate.
Octadecyl 3,5-di-tert -butyl-4-hydroxyhydrocinnamate (CAS Reg. No. 2082-79-3): For use only: (1) At levels not exceeding 0.2 percent by weight in semirigid and rigid acrylic and modified acrylic plastics, where the finished articles contact foods containing not more than 15 percent alcohol; and (2) at levels not exceeding 0.01 percent by weight in semirigid and rigid acrylic and modified acrylic plastics intended for repeated food-contact use where the finished article may be used for foods containing more than 15 percent alcohol.
Phenyl salicylate.
(6) Release agents: Fatty acids derived from animal and vegetable fats and oils, and fatty alcohols derived from such acids.
(7) Surface active agent: Sodium dodecylbenzenesulfonate.
(8) Miscellaneous materials:
Di(2-ethylhexyl) phthalate, for use only as a flow promoter at a level not to exceed 3 weight-percent based on the monomers.
Oxalic acid, for use only as a polymerization catalyst aid.
Tetraethylenepentamine, for use only as a catalyst activator at a level not to exceed 0.5 weight-percent based on the monomers.
Toluene.
Xylene.
(b) The semirigid and rigid acrylic and modified acrylic plastics, in the finished form in which they are to contact food, when extracted with the solvent or solvents characterizing the type of food and under the conditions of time and temperature as determined from tables 1 and 2 of § 176.170(c) of this chapter, shall yield extractives not to exceed the following, when tested by the methods prescribed in paragraph (c) of this section. The acrylic and modified acrylic polymers or plastics intended to be used as components of articles also shall yield extractives not to exceed the following limitations when prepared as strips as described in paragraph (c)(2) of this section:
(1) Total nonvolatile extractives not to exceed 0.3 milligram per square inch of surface tested.
(2) Potassium permanganate oxidizable distilled water and 8 and 50 percent alcohol extractives not to exceed an absorbance of 0.15.
(3) Ultraviolet-absorbing distilled water and 8 and 50 percent alcohol extractives not to exceed an absorbance of 0.30.
(4) Ultraviolet-absorbing n- heptane extractives not to exceed an absorbance of 0.10.
(c) Analytical methods - (1) Selection of extractability conditions. These are to be chosen as provided in § 176.170(c) of this chapter.
(2) Preparation of samples. Sufficient samples to allow duplicates of all applicable tests shall be cut from the articles or formed from the plastic composition under tests, as strips about 2.5 inches by about 0.85-inch wide by about 0.125-inch thick. The total exposed surface should be 5 square inches +/-0.5-square inch. The samples, after preparation, shall be washed with a clean brush under hot tapwater, rinsed under running hot tapwater (140 deg.F minimum), rinsed with distilled water, and air-dried in a dust-free area or in a desiccator.
(3) Preparation of solvents. The water used shall be double-distilled water, prepared in a still using a block tin condenser. The 8 and 50 percent (by volume) alcohol solvents shall be prepared from ethyl alcohol meeting the specifications of the United States Pharmacopeia XX and diluted with double-distilled water that has been prepared in a still using a tin block condenser. The n -heptane shall be spectrophotometric grade. Adequate precautions must be taken to keep all solvents dust-free.
(4) Blank values on solvents. (i) Duplicate determinations of residual solids shall be run on samples of each solvent that have been exposed to the temperature-time conditions of the extraction test without the plastic sample. Sixty milliliters of exposed solvent is pipetted into a clean, weighed platinum dish, evaporated to 2-5 milliliters on a nonsparking, low-temperature hot plate and dried in 212 deg.F oven for 30 minutes. The residue for each solvent shall be determined by weight and the average residue weight used as the blank value in the total solids determination set out in paragraph (c)(6) of this section. The residue for an acceptable solvent sample shall not exceed 0.5 milligram per 60 milliliters.
(ii) For acceptability in the ultraviolet absorbers test, a sample of each solvent shall be scanned in an ultraviolet spectrophotometer in 5-centimeter silica spectrophotometric absorption cells. The absorbance of the distilled water when measured versus air in the reference cell shall not exceed 0.03 at any point in the wavelength region of 245 to 310 m[micro]. The absorbance of the 8 percent alcohol when measured versus distilled water in the reference cell shall not exceed 0.01 at any point in the wavelength region of 245 to 310 m[micro]. The absorbance of the 50 percent alcohol when measured versus distilled water in the reference cell shall not exceed 0.05 at any point in the wavelength region of 245 to 310 m[micro]. The absorbance of the heptane when measured versus distilled water in the reference cell shall not exceed 0.15 at 245, 0.09 at 260, 0.04 at 270, and 0.02 at any point in the wavelength region of 280 to 310 m[micro].
(iii) Duplicate ultraviolet blank determinations shall be run on samples of each solvent that has been exposed to the temperature-time conditions of the extraction test without the plastic sample. An aliquot of the exposed solvent shall be measured versus the unexposed solvent in the reference cell. The average difference in the absorbances at any wavelength in the region of 245 to 310 m[micro] shall be used as a blank correction for the ultraviolet absorbers measured at the same wavelength according to paragraph (c)(8)(ii) of this section.
(iv) The acceptability of the solvents for use in the permanganate test shall be determined by preparing duplicate permanganate test blanks according to paragraph (c)(7)(iv) of this section. For this test, the directions referring to the sample extract shall be disregarded. The blanks shall be scanned in 5-centimeter silica spectrophotometric cells in the spectrophotometer versus the appropriate solvent as reference. The absorbance in distilled water in the wavelength region of 544 to 552 m[micro] should be 1.16 but must not be less than 1.05 nor more than 1.25. The absorbance in the 8 and 50 percent alcohol must not be less than 0.85 nor more than 1.15.
(v) Duplicate permanganate test determinations shall be run on samples of distilled water and 8 and 50 percent alcohol solvents that have been exposed to the temperature-time conditions of the extraction test without the plastic sample. The procedure shall be as described in paragraph (c)(7)(iv) of this section, except that the appropriate exposed solvent shall be substituted where the directions call for sample extract. The average difference in the absorbances in the region of 544 to 552 m[micro] shall be used as a blank correction for the determination of permanganate oxidizable extractives according to paragraph (c)(7)(iv) of this section.
(5) Extraction procedure. For each extraction, place a plastic sample in a clean 25 millimeters * 200 millimeters hard-glass test tube and add solvent equal to 10 milliliters of solvent per square inch of plastic surface. This amount will be between 45 milliliters and 55 milliliters. The solvent must be preequilibrated to the temperature of the extraction test. Close the test tube with a ground-glass stopper and expose to the specified temperature for the specified time. Cool the tube and contents to room temperature if necessary.
(6) Determination of total nonvolatile extractives. Remove the plastic strip from the solvent with a pair of clean forceps and wash the strip with 5 milliliters of the appropriate solvent, adding the washings to the contents of the test tube. Pour the contents of the test tube into a clean, weighed platinum dish. Wash the tube with 5 milliliters of the appropriate solvent and add the solvent to the platinum dish. Evaporate the solvent to 2-5 milliliters on a nonsparking, low-temperature hotplate. Complete the evaporation in a 212 deg.F oven for 30 minutes. Cool the dish in a desiccator for 30 minutes and weigh to the nearest 0.1 milligram. Calculate the total nonvolatile extractives as follows:

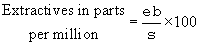
where:
e = Total increase in weight of the dish, in milligrams.
b = Blank value of the solvent in milligrams, as determined in paragraph (c)(4)(i) of this section.
s = Total surface of the plastic sample in square inches.
(7) Determination of potassium permanganate oxidizable extractives. (i) Pipette 25 milliliters of distilled water into a clean 125-milliliter Erlenmeyer flask that has been rinsed several times with aliquots of distilled water. This is the blank. Prepare a distilled water solution containing 1.0 part per million of p- methoxyphenol (melting point 54-56 deg.C, Eastman grade or equivalent). Pipette 25 milliliters of this p- methoxyphenol solution into a rinsed Erlenmeyer flask. Pipette exactly 3.0 milliliters of 154 parts per million aqueous potassium permanganate solution into the p- methoxyphenol and exactly 3.0 milliliters into the blank, in that order. Swirl both flasks to mix the contents and then transfer aliquots from each flask into matched 5-centimeter spectrophotometric absorption cells. The cells are placed in the spectrophotometer cell compartment with the p- methoxyphenol solution in the reference beam. Spectrophotometric measurement is conducted as in paragraph (c)(7)(iv) of this section. The absorbance reading in the region 544-552 m[micro] should be 0.24 but must be not less than 0.12 nor more than 0.36. This test shall be run in duplicate. For the purpose of ascertaining compliance with the limitations in paragraph (b)(2) of this section, the absorbance measurements obtained on the distilled water extracts according to paragraph (c)(7)(iv) of this section shall be multiplied by a correction factor, calculated as follows:
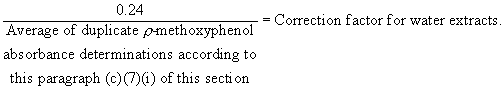
(ii) The procedure in paragraph (c)(7)(i) of this section is repeated except that, in this instance, the solvent shall be 8 percent alcohol. The absorbance in the region 544-552 m[micro] should be 0.26 but must be not less than 0.13 nor more than 0.39. This test shall be run in duplicate. For the purpose of ascertaining compliance with the limitations prescribed in paragraph (b)(2) of this section, the absorbance measurements obtained on the 8 percent alcohol extracts according to paragraph (c)(7)(iv) of this section shall be multiplied by a correction factor, calculated as follows:
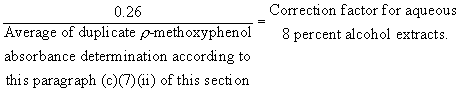
(iii) The procedure in paragraph (c)(7)(i) of this section is repeated except that, in this instance, the solvent shall be 50 percent alcohol. The absorbance in the region 544-552 m[micro] should be 0.25 but must be not less than 0.12 nor more than 0.38. This test shall be run in duplicate. For the purpose of ascertaining compliance with the limitations prescribed in paragraph (b)(2) of this section, the absorbance measurements obtained on the 50 percent alcohol extracts according to paragraph (c)(7)(iv) of this section shall be multiplied by a correction factor, calculated as follows:
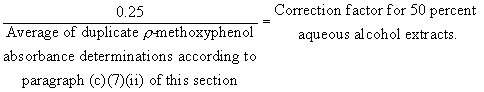
(iv) Water and 8 and 50 percent alcohol extracts. Pipette 25 milliliters of the appropriate solvent into a clean, 125-milliliter Erlenmeyer flask that has been rinsed several times with aliquots of the same solvent. This is the blank. Into another similarly rinsed flask, pipette 25 milliliters of the sample extract that has been exposed under the conditions specified in paragraph (c)(5) of this section. Pipette exactly 3.0 milliliters of 154 parts per million aqueous potassium permanganate solution into the sample and exactly 3.0 milliliters into the blank, in that order. Before use, the potassium permanganate solution shall be checked as in paragraph (c)(7)(i) of this section. Both flasks are swirled to mix the contents, and then aliquots from each flask are transferred to matched 5-centimeter spectrophotometric absorption cells. Both cells are placed in the spectrophotometer cell compartment with the sample solution in the reference beam. The spectrophotometer is adjusted for 0 and 100 percent transmittance at 700 m[micro]. The spectrum is scanned on the absorbance scale from 700 m[micro] to 500 m[micro] in such a way that the region 544 m[micro] to 552 m[micro] is scanned within 5 minutes to 10 minutes of the time that permanganate was added to the solutions. The height of the absorbance peak shall be measured, corrected for the blank as determined in paragraph (c)(4)(v) of this section, and multiplied by the appropriate correction factor determined according to paragraph (c)(7)(i), (ii), and (iii) of this section. This test shall be run in duplicate and the two results averaged.
(8) Determination of ultraviolet-absorbing extractives. (i) A distilled water solution containing 1.0 part per million of p- methoxyphenol (melting point 54 deg.C-56 deg.C. Eastman grade or equivalent) shall be scanned in the region 360 to 220 m[micro] in 5-centimeter silica spectrophotometric absorption cells versus a distilled water reference. The absorbance at the wavelength of maximum absorbance (should be about 285 m[micro]) is about 0.11 but must be not less than 0.08 nor more than 0.14. This test shall be run in duplicate. For the purpose of ascertaining compliance with the limitations prescribed in paragraph (b)(3) and (4) of this section, the absorbance obtained on the extracts according to paragraph (c)(8)(ii) of this section shall be multiplied by a correction factor, calculated as follows:
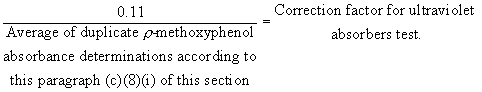
(ii) An aliquot of the extract that has been exposed under the conditions specified in paragraph (c)(5) of this section is scanned in the wavelength region 360 to 220 m[micro] versus the appropriate solvent reference in matched 5-centimeter silica spectrophotometric absorption cells. The height of any absorption peak shall be measured, corrected for the blank as determined in paragraph (c)(4)(iii) of this section, and multiplied by the correction factor determined according to paragraph (c)(8)(i) of this section.
(d) In accordance with current good manufacturing practice, finished semirigid and rigid acrylic and modified acrylic plastics, and articles containing these polymers, intended for repeated use in contact with food shall be thoroughly cleansed prior to their first use in contact with food.
(e) Acrylonitrile copolymers identified in this section shall comply with the provisions of § 180.22 of this chapter.
(f) The acrylic and modified acrylic polymers identified in and complying with this section, when used as components of the food-contact surface of an article that is the subject of a regulation in this part and in parts 174, 175, 176, and 178 of this chapter, shall comply with any specifications and limitations prescribed by such regulation for the article in the finished form in which it is to contact food.
[42 FR 14572, Mar. 15, 1977; 42 FR 56728, Oct. 28, 1977, as amended at 43 FR 54927, Nov. 24, 1978; 45 FR 67320, Oct. 10, 1980; 46 FR 46796, Sept. 22, 1981; 49 FR 10108, Mar. 19, 1984; 49 FR 13139, Apr. 3, 1984; 50 FR 31045, July 24, 1985; 87 FR 31089, May 20, 2022]
|