|
The food additive poloxalene may be safely used in accordance with the following prescribed conditions:
(a) The additive consists of polyoxy-propylene-polyoxyethylene glycol non-ionic block polymer meeting the following specifications:
(1) Molecular weight range: 2,850-3,150.
(2) Hydroxyl number: 35.7-39.4.
(3) Cloud point (10 percent solution): 42 deg.C-46 deg.C.
(4) Structural formula:
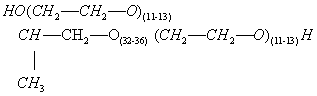
(b) In feed as a surfactant for the flaking of feed grains when added to liquid grain conditioner in an amount not to exceed 1.0 percent of the conditioner. The conditioner is added to the feed at a rate of 1 quart per ton of feed.
(c) The label and labeling shall bear, in addition to the other information required by the Act:
(1) The name of the additive.
(2) Adequate directions and warnings for use.
|